当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Programmed co-delivery of platinum nanodrugs and gemcitabine by a clustered nanocarrier for precision chemotherapy for NSCLC tumors.
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2019-12-11 , DOI: 10.1039/c9tb02055a Huihui Shi 1 , Ming Xu 2 , Jianhua Zhu 3 , Yang Li 3 , Zhiyu He 4 , Yuxia Zhang 3 , Qunwei Xu 3 , Yimin Niu 5 , Yang Liu 3
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2019-12-11 , DOI: 10.1039/c9tb02055a Huihui Shi 1 , Ming Xu 2 , Jianhua Zhu 3 , Yang Li 3 , Zhiyu He 4 , Yuxia Zhang 3 , Qunwei Xu 3 , Yimin Niu 5 , Yang Liu 3
Affiliation
|
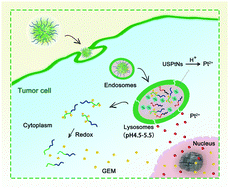
Recently, ultra-small platinum nanoparticles (USPtNs) have been found that can kill cancer cells by leaching Pt ions into acidic organelles, such as cell endosomes or lysosomes. Unfortunately, tumor-specific accumulation is difficult to achieve with such platinum nanodrugs of less than 5 nm due to their short half-life in vivo and broad range of toxicity to normal tissues. Programmable multi-drug release for combinational chemotherapy by hierarchical nanostructures provides a promising solution for cancer-targeted therapy. Herein, we demonstrated a pH/redox dual stimuli-responsive clustered nanoparticle as a vehicle for simultaneously delivering USPtNs and gemcitabine (GEM) to treat non-small-cell lung cancer. The clustered nanoparticle (denoted as GP-NA) was composed of disulfide-bond-containing GEM-grafted copolymers (PEG-b-P(LL-g-GEM)), pH-sensitive polypeptides (OAPI), and USPtNs. Such a hybrid nanosystem completes multiple tasks inside cancer cells, which include the generation of cytotoxic Pt ions in response to lysosomal acidic environments and the subsequent release of GEM in cytoplasmic reduction environments. Compared with non-acid-sensitive nanoparticles or free drugs, GP-NA exhibited cumulative and enhanced anti-tumor efficacy in vivo, which may be attributed to the simultaneous inhibition of ribonucleotide reductase and DNA replication in nuclei by the GEM and Pt ions. Together, our work provides a promising strategy in the co-delivery of USPtNs and GEM for precision cancer chemotherapy.
中文翻译:

通过簇状纳米载体以编程方式共同递送铂纳米药物和吉西他滨,用于NSCLC肿瘤的精确化疗。
最近,发现了超小铂纳米颗粒(USPtNs),可以通过将Pt离子浸入酸性细胞器(例如细胞内体或溶酶体)中杀死癌细胞。不幸的是,由于此类铂纳米药物在体内的半衰期短且对正常组织的毒性范围广,因此使用小于5 nm的铂纳米药物难以实现肿瘤特异性的蓄积。通过分级纳米结构进行组合化疗的可编程多药释放为癌症靶向治疗提供了有希望的解决方案。在本文中,我们证明了pH /氧化还原双重刺激响应性簇状纳米颗粒作为媒介物,可同时递送USPtNs和吉西他滨(GEM)治疗非小细胞肺癌。簇状纳米颗粒(表示为GP-NA)由含二硫键的GEM接枝共聚物(PEG-bP(LL-g-GEM))组成,pH敏感多肽(OAPI)和USPtN。这种杂交纳米系统完成了癌细胞内部的多项任务,其中包括响应溶酶体酸性环境而产生细胞毒性Pt离子,以及随后在细胞质还原环境中释放GEM。与非酸敏感性纳米粒子或游离药物相比,GP-NA在体内表现出累积的和增强的抗肿瘤功效,这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和核内DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM的共同交付提供了一种有前途的策略。其中包括响应溶酶体酸性环境而产生细胞毒性Pt离子,以及随后在胞质还原环境中释放GEM。与非酸敏感性纳米粒子或游离药物相比,GP-NA在体内表现出累积的和增强的抗肿瘤功效,这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和核内DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM的共同交付提供了一种有前途的策略。其中包括响应溶酶体酸性环境而产生细胞毒性Pt离子,以及随后在胞质还原环境中释放GEM。与非酸敏感性纳米粒子或游离药物相比,GP-NA在体内表现出累积的和增强的抗肿瘤功效,这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和核内DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM的共同交付提供了一种有前途的策略。这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和细胞核中的DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM共同交付提供了一种有前途的策略。这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和细胞核中的DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM的共同交付提供了一种有前途的策略。
更新日期:2020-01-02
中文翻译:

通过簇状纳米载体以编程方式共同递送铂纳米药物和吉西他滨,用于NSCLC肿瘤的精确化疗。
最近,发现了超小铂纳米颗粒(USPtNs),可以通过将Pt离子浸入酸性细胞器(例如细胞内体或溶酶体)中杀死癌细胞。不幸的是,由于此类铂纳米药物在体内的半衰期短且对正常组织的毒性范围广,因此使用小于5 nm的铂纳米药物难以实现肿瘤特异性的蓄积。通过分级纳米结构进行组合化疗的可编程多药释放为癌症靶向治疗提供了有希望的解决方案。在本文中,我们证明了pH /氧化还原双重刺激响应性簇状纳米颗粒作为媒介物,可同时递送USPtNs和吉西他滨(GEM)治疗非小细胞肺癌。簇状纳米颗粒(表示为GP-NA)由含二硫键的GEM接枝共聚物(PEG-bP(LL-g-GEM))组成,pH敏感多肽(OAPI)和USPtN。这种杂交纳米系统完成了癌细胞内部的多项任务,其中包括响应溶酶体酸性环境而产生细胞毒性Pt离子,以及随后在细胞质还原环境中释放GEM。与非酸敏感性纳米粒子或游离药物相比,GP-NA在体内表现出累积的和增强的抗肿瘤功效,这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和核内DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM的共同交付提供了一种有前途的策略。其中包括响应溶酶体酸性环境而产生细胞毒性Pt离子,以及随后在胞质还原环境中释放GEM。与非酸敏感性纳米粒子或游离药物相比,GP-NA在体内表现出累积的和增强的抗肿瘤功效,这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和核内DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM的共同交付提供了一种有前途的策略。其中包括响应溶酶体酸性环境而产生细胞毒性Pt离子,以及随后在胞质还原环境中释放GEM。与非酸敏感性纳米粒子或游离药物相比,GP-NA在体内表现出累积的和增强的抗肿瘤功效,这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和核内DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM的共同交付提供了一种有前途的策略。这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和细胞核中的DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM共同交付提供了一种有前途的策略。这可能归因于GEM和Pt离子同时抑制核糖核苷酸还原酶和细胞核中的DNA复制。在一起,我们的工作为精确癌症化学治疗的USPtNs和GEM的共同交付提供了一种有前途的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号