当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Micrococcal-Nuclease-Triggered On-Demand Release of Vancomycin from Intramedullary Implant Coating Eradicates Staphylococcus aureus Infection in Mouse Femoral Canals.
ACS Central Science ( IF 12.7 ) Pub Date : 2019-12-10 , DOI: 10.1021/acscentsci.9b00870 Ananta Ghimire 1 , Jordan D Skelly 1 , Jie Song 1
ACS Central Science ( IF 12.7 ) Pub Date : 2019-12-10 , DOI: 10.1021/acscentsci.9b00870 Ananta Ghimire 1 , Jordan D Skelly 1 , Jie Song 1
Affiliation
|
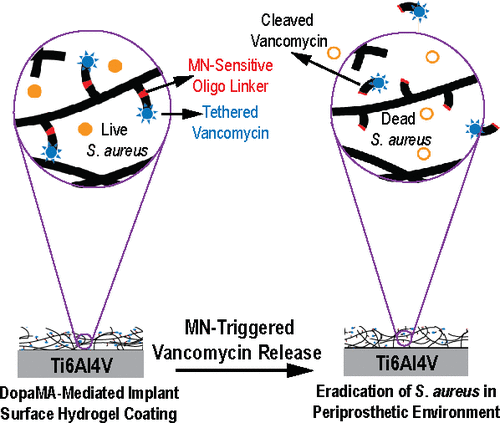
Preventing orthopedic implant-associated bacterial infections remains a critical challenge. Current practices of physically blending high-dose antibiotics with bone cements is known for cytotoxicity while covalently tethering antibiotics to implant surfaces is ineffective in eradicating bacteria from the periprosthetic tissue environment due to the short-range bactericidal actions, which are limited to the implant surface. Here, we covalently functionalize poly(ethylene glycol) dimethacrylate hydrogel coatings with vancomycin via an oligonucleotide linker sensitive to Staphylococcus aureus (S. aureus) micrococcal nuclease (MN) (PEGDMA-Oligo-Vanco). This design enables the timely release of vancomycin in the presence of S. aureus to kill the bacteria both on the implant surface and within the periprosthetic tissue environment. Ti6Al4V intramedullary (IM) pins surface-tethered with dopamine methacrylamide (DopaMA) and uniformly coated with PEGDMA-Oligo-Vanco effectively prevented periprosthetic infections in mouse femoral canals inoculated with bioluminescent S. aureus. Longitudinal bioluminescence monitoring, μCT quantification of femoral bone changes, end point quantification of implant surface bacteria, and histological detection of S. aureus in the periprosthetic tissue environment confirmed rapid and sustained bacterial clearance by the PEGDMA-Oligo-Vanco coating. The observed eradication of bacteria was in stark contrast with the significant bacterial colonization on implants and osteomyelitis development found in the absence of the MN-sensitive bactericidal coating. The effective vancomycin tethering dose presented in this on-demand release strategy was >200 times lower than the typical prophylactic antibiotic contents used in bone cements and may be applied to medical implants and bone/dental cements to prevent periprosthetic infections in high-risk clinical scenarios. This study also supports the timely bactericidal action by MN-triggered release of antibiotics as an effective prophylactic method to bypass the notoriously harder to treat periprosthetic biofilms and osteomyelitis.
中文翻译:

微球菌核酸酶触发的髓内植入物涂层按需释放万古霉素可根除小鼠股管中的金黄色葡萄球菌感染。
预防骨科植入物相关的细菌感染仍然是一个严峻的挑战。目前将高剂量抗生素与骨水泥物理混合的做法因细胞毒性而闻名,而将抗生素共价连接到植入物表面,由于其短程杀菌作用仅限于植入物表面,因此无法有效根除假体周围组织环境中的细菌。在这里,我们通过对金黄色葡萄球菌(S. aureus)微球菌核酸酶(MN)敏感的寡核苷酸连接体与万古霉素共价功能化聚(乙二醇)二甲基丙烯酸酯水凝胶涂层(PEGDMA-Oligo-Vanco)。这种设计使得万古霉素能够在金黄色葡萄球菌存在的情况下及时释放,以杀死种植体表面和假体周围组织环境内的细菌。Ti6Al4V 髓内 (IM) 针表面拴有多巴胺甲基丙烯酰胺 (DopaMA),并均匀涂有 PEGDMA-Oligo-Vanco,可有效预防接种生物发光金黄色葡萄球菌的小鼠股管假体周围感染。纵向生物发光监测、股骨变化的 μCT 定量、植入物表面细菌的终点定量以及假体周围组织环境中金黄色葡萄球菌的组织学检测证实了 PEGDMA-Oligo-Vanco 涂层能够快速、持续地清除细菌。观察到的细菌根除与在没有微核敏感杀菌涂层的情况下发现的植入物上显着的细菌定植和骨髓炎的发展形成鲜明对比。这种按需释放策略中提出的有效万古霉素束缚剂量比骨水泥中使用的典型预防性抗生素含量低 200 倍以上,可应用于医用植入物和骨/牙科水泥,以预防高风险临床情况下的假体周围感染。这项研究还支持微球触发抗生素释放的及时杀菌作用,作为一种有效的预防方法,可以绕过众所周知的难以治疗的假体周围生物膜和骨髓炎。
更新日期:2019-12-27
中文翻译:

微球菌核酸酶触发的髓内植入物涂层按需释放万古霉素可根除小鼠股管中的金黄色葡萄球菌感染。
预防骨科植入物相关的细菌感染仍然是一个严峻的挑战。目前将高剂量抗生素与骨水泥物理混合的做法因细胞毒性而闻名,而将抗生素共价连接到植入物表面,由于其短程杀菌作用仅限于植入物表面,因此无法有效根除假体周围组织环境中的细菌。在这里,我们通过对金黄色葡萄球菌(S. aureus)微球菌核酸酶(MN)敏感的寡核苷酸连接体与万古霉素共价功能化聚(乙二醇)二甲基丙烯酸酯水凝胶涂层(PEGDMA-Oligo-Vanco)。这种设计使得万古霉素能够在金黄色葡萄球菌存在的情况下及时释放,以杀死种植体表面和假体周围组织环境内的细菌。Ti6Al4V 髓内 (IM) 针表面拴有多巴胺甲基丙烯酰胺 (DopaMA),并均匀涂有 PEGDMA-Oligo-Vanco,可有效预防接种生物发光金黄色葡萄球菌的小鼠股管假体周围感染。纵向生物发光监测、股骨变化的 μCT 定量、植入物表面细菌的终点定量以及假体周围组织环境中金黄色葡萄球菌的组织学检测证实了 PEGDMA-Oligo-Vanco 涂层能够快速、持续地清除细菌。观察到的细菌根除与在没有微核敏感杀菌涂层的情况下发现的植入物上显着的细菌定植和骨髓炎的发展形成鲜明对比。这种按需释放策略中提出的有效万古霉素束缚剂量比骨水泥中使用的典型预防性抗生素含量低 200 倍以上,可应用于医用植入物和骨/牙科水泥,以预防高风险临床情况下的假体周围感染。这项研究还支持微球触发抗生素释放的及时杀菌作用,作为一种有效的预防方法,可以绕过众所周知的难以治疗的假体周围生物膜和骨髓炎。











































 京公网安备 11010802027423号
京公网安备 11010802027423号