当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deamidation and Enzymatic Hydrolysis of Gliadins Alter Their Processing by Dendritic Cells in Vitro.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jafc.9b06075 Clélia Villemin 1 , Olivier Tranquet 1 , Véronique Solé-Jamault 1 , Joost J Smit 2 , Raymond H H Pieters 2 , Sandra Denery-Papini 1 , Grégory Bouchaud 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jafc.9b06075 Clélia Villemin 1 , Olivier Tranquet 1 , Véronique Solé-Jamault 1 , Joost J Smit 2 , Raymond H H Pieters 2 , Sandra Denery-Papini 1 , Grégory Bouchaud 1
Affiliation
|
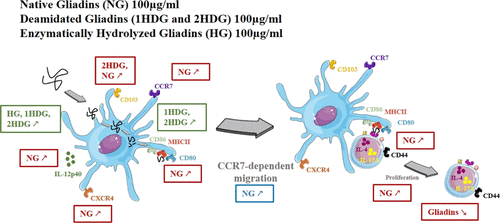
Gliadins are major wheat allergens. Their treatment by acid or enzymatic hydrolysis has been shown to modify their allergenic potential. As the interaction of food proteins with dendritic cells (DCs) is a key event in allergic sensitization, we wished to investigate whether deamidation and enzymatic hydrolysis influence gliadin processing by DC and to examine the capacity of gliadins to activate DCs. We compared the uptake and degradation of native and modified gliadins by DCs using mouse bone marrow-derived DCs. We also analyzed the effects of these interactions on the phenotypes of DCs and T helper (Th) lymphocytes. Modifying gliadins induced a change in physicochemical properties (molecular weight, hydrophobicity, and sequence) and also in the peptide size. These alterations in turn led to increased uptake and intracellular degradation of the proteins by DCs. Native gliadins (NGs) (100 μg/mL), but not modified gliadins, increased the frequency of DC expressing CD80 (15.41 ± 2.36% vs 6.81 ± 1.10%, p < 0.001), CCR7 (28.53 ± 8.17% vs 17.88 ± 2.53%, p < 0.001), CXCR4 (70.14 ± 4.63% vs 42.82 ± 1.96%, p < 0.001), and CCR7-dependent migration (2.46 ± 1.45 vs 1.00 ± 0.22, p < 0.01) compared with NGs. This was accompanied by Th lymphocyte activation (30.37 ± 3.87% vs 21.53 ± 3.14%, p < 0.1) and proliferation (16.39 ± 3.97% vs 9.31 ± 2.80%, p > 0.1). Moreover, hydrolysis decreases the peptide size and induces an increase in gliadin uptake and degradation. Deamidation and extensive enzymatic hydrolysis of gliadins modify their interaction with DCs, leading to alteration of their immunostimulatory capacity. These findings demonstrate the strong relationship between the biochemical characteristics of proteins and immune cell interactions.
中文翻译:

醇溶蛋白的脱酰胺和酶水解改变了树突状细胞的体外加工过程。
麦醇溶蛋白是主要的小麦过敏原。通过酸或酶水解对其进行处理已显示出可改变其致敏潜力。由于食物蛋白与树突状细胞(DC)的相互作用是变态反应致敏的关键事件,因此我们希望调查脱酰胺作用和酶水解是否影响DC对麦醇溶蛋白的加工,并研究麦醇溶蛋白激活DC的能力。我们比较了使用小鼠骨髓来源的DC摄取的DC对天然和修饰的麦醇溶蛋白的摄取和降解。我们还分析了这些相互作用对DC和T辅助(Th)淋巴细胞表型的影响。修饰麦醇溶蛋白诱导了理化性质(分子量,疏水性和序列)以及肽大小的改变。这些改变反过来导致DC摄取蛋白质增加和细胞内降解。天然麦醇溶蛋白(NGs)(100μg/ mL)而非修饰的麦醇溶蛋白增加了DC表达CD80的频率(15.41±2.36%vs 6.81±1.10%,p <0.001),CCR7(28.53±8.17%vs 17.88±2.53) %,p <0.001),CXCR4(70.14±4.63%vs 42.82±1.96%,p <0.001)和CCR7依赖的迁移(2.46±1.45 vs 1.00±0.22,p <0.01)。伴随有Th淋巴细胞活化(30.37±3.87%vs 21.53±3.14%,p <0.1)和增殖(16.39±3.97%vs 9.31±2.80%,p> 0.1)。此外,水解减小了肽的大小,并引起了麦醇溶蛋白摄取和降解的增加。麦醇溶蛋白的脱酰胺作用和广泛的酶水解作用改变了它们与DC的相互作用,导致其免疫刺激能力改变。这些发现证明了蛋白质的生化特性与免疫细胞相互作用之间的密切关系。
更新日期:2020-01-23
中文翻译:

醇溶蛋白的脱酰胺和酶水解改变了树突状细胞的体外加工过程。
麦醇溶蛋白是主要的小麦过敏原。通过酸或酶水解对其进行处理已显示出可改变其致敏潜力。由于食物蛋白与树突状细胞(DC)的相互作用是变态反应致敏的关键事件,因此我们希望调查脱酰胺作用和酶水解是否影响DC对麦醇溶蛋白的加工,并研究麦醇溶蛋白激活DC的能力。我们比较了使用小鼠骨髓来源的DC摄取的DC对天然和修饰的麦醇溶蛋白的摄取和降解。我们还分析了这些相互作用对DC和T辅助(Th)淋巴细胞表型的影响。修饰麦醇溶蛋白诱导了理化性质(分子量,疏水性和序列)以及肽大小的改变。这些改变反过来导致DC摄取蛋白质增加和细胞内降解。天然麦醇溶蛋白(NGs)(100μg/ mL)而非修饰的麦醇溶蛋白增加了DC表达CD80的频率(15.41±2.36%vs 6.81±1.10%,p <0.001),CCR7(28.53±8.17%vs 17.88±2.53) %,p <0.001),CXCR4(70.14±4.63%vs 42.82±1.96%,p <0.001)和CCR7依赖的迁移(2.46±1.45 vs 1.00±0.22,p <0.01)。伴随有Th淋巴细胞活化(30.37±3.87%vs 21.53±3.14%,p <0.1)和增殖(16.39±3.97%vs 9.31±2.80%,p> 0.1)。此外,水解减小了肽的大小,并引起了麦醇溶蛋白摄取和降解的增加。麦醇溶蛋白的脱酰胺作用和广泛的酶水解作用改变了它们与DC的相互作用,导致其免疫刺激能力改变。这些发现证明了蛋白质的生化特性与免疫细胞相互作用之间的密切关系。







































 京公网安备 11010802027423号
京公网安备 11010802027423号