当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Novel Oxotriazinoindole Inhibitors of Aldose Reductase: Isosteric Sulfur/Oxygen Replacement in the Thioxotriazinoindole Cemtirestat Markedly Improved Inhibition Selectivity.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.jmedchem.9b01747 Matúš Hlaváč 1 , Lucia Kováčiková 2 , Marta Šoltésová Prnová 2 , Peter Šramel 1 , Gabriela Addová 3 , Magdaléna Májeková 2 , Gilles Hanquet 4 , Andrej Boháč 1, 5 , Milan Štefek 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.jmedchem.9b01747 Matúš Hlaváč 1 , Lucia Kováčiková 2 , Marta Šoltésová Prnová 2 , Peter Šramel 1 , Gabriela Addová 3 , Magdaléna Májeková 2 , Gilles Hanquet 4 , Andrej Boháč 1, 5 , Milan Štefek 2
Affiliation
|
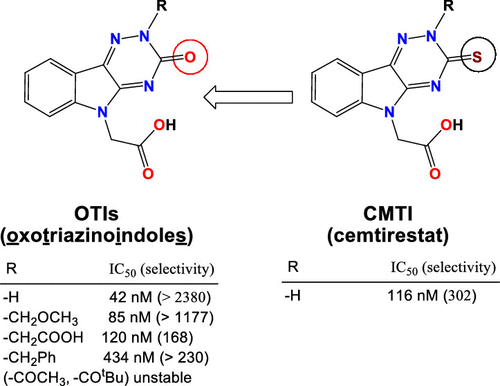
Inhibition of aldose reductase (AR), the first enzyme of the polyol pathway, is a promising approach in treatment of diabetic complications. We proceeded with optimization of the thioxotriazinoindole scaffold of the novel AR inhibitor cemtirestat by replacement of sulfur with oxygen. A series of 2-(3-oxo-2H-[1,2,4]triazino[5,6-b]indol-5(3H)-yl)acetic acid derivatives (OTIs), designed by molecular modeling and docking, were synthesized. More electronegative and less bulky oxygen of OTIs compared to the sulfur of the original thioxotriazinoindole congeners was found to form a stronger H-bond with Leu300 of AR and to render larger rotational flexibility of the carboxymethyl pharmacophore. AR inhibitory activities of the novel compounds were characterized by the IC50 values in a submicromolar range. Markedly enhanced inhibition selectivity relative to the structurally related aldehyde reductase was recorded. To conclude, structure modification of the original carboxymethylated thioxotriazinoindole cemtirestat by isosteric replacement of sulfur with oxygen in combination with variable N(2) simple substituents provided novel analogues with increased AR inhibition efficacy and markedly improved selectivity.
中文翻译:

醛糖还原酶的新型氧代三嗪并吲哚抑制剂的开发:硫代三唑并吲哚西替司他中的等位硫/氧替代显着提高了抑制选择性。
抑制醛糖还原酶(AR),多元醇途径的第一种酶,是治疗糖尿病并发症的一种有前途的方法。我们通过用氧气替代硫来优化新型AR抑制剂西非司他的硫代三嗪并吲哚骨架。通过分子建模和对接设计了一系列2-(3-oxo-2H- [1,2,4] triazino [5,6-b]吲哚-5(3H)-基)乙酸衍生物(OTI),被合成。与原始的硫代三嗪并吲哚同类物的硫相比,OTI的负电性更高且体积较小的氧被发现与AR的Leu300形成更强的H键,并使羧甲基药效团具有更大的旋转柔韧性。通过在亚微摩尔范围内的IC 50值来表征新化合物的AR抑制活性。记录了相对于结构相关的醛还原酶的抑制选择性显着提高。综上所述,通过用氧与可变N(2)简单取代基进行氧的硫的等位取代,对原始羧甲基化硫代三嗪并吲哚西替司他的结构修饰提供了新的类似物,其AR抑制作用增强,选择性显着提高。
更新日期:2019-12-30
中文翻译:

醛糖还原酶的新型氧代三嗪并吲哚抑制剂的开发:硫代三唑并吲哚西替司他中的等位硫/氧替代显着提高了抑制选择性。
抑制醛糖还原酶(AR),多元醇途径的第一种酶,是治疗糖尿病并发症的一种有前途的方法。我们通过用氧气替代硫来优化新型AR抑制剂西非司他的硫代三嗪并吲哚骨架。通过分子建模和对接设计了一系列2-(3-oxo-2H- [1,2,4] triazino [5,6-b]吲哚-5(3H)-基)乙酸衍生物(OTI),被合成。与原始的硫代三嗪并吲哚同类物的硫相比,OTI的负电性更高且体积较小的氧被发现与AR的Leu300形成更强的H键,并使羧甲基药效团具有更大的旋转柔韧性。通过在亚微摩尔范围内的IC 50值来表征新化合物的AR抑制活性。记录了相对于结构相关的醛还原酶的抑制选择性显着提高。综上所述,通过用氧与可变N(2)简单取代基进行氧的硫的等位取代,对原始羧甲基化硫代三嗪并吲哚西替司他的结构修饰提供了新的类似物,其AR抑制作用增强,选择性显着提高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号