当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using Multiple Analytical Platforms to Investigate the Androgen Depletion Effects on Fecal Metabolites in a Mouse Model of Systemic Lupus Erythematosus.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jproteome.9b00558 Fang Yuan , James Harder , Jing Ma , Xinmin Yin , Xiang Zhang , Michele M. Kosiewicz
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jproteome.9b00558 Fang Yuan , James Harder , Jing Ma , Xinmin Yin , Xiang Zhang , Michele M. Kosiewicz
|
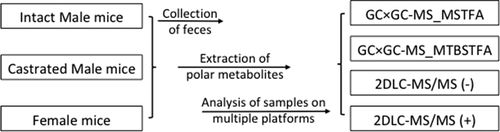
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by circulating autoantibodies that deposit in target organs (e.g., kidneys), resulting in chronic inflammation and eventual destruction of the organ. SLE is much more prevalent in females than males in both humans and spontaneous mouse models of lupus, such as NZBxNZW F1 (BWF1) mice. Depleting androgens by castration dramatically increases the susceptibility of BWF1 male to lupus. We compared fecal metabolite profiles of castrated BWF1 (androgen-depleted) male, intact (androgen-replete) male, and female mice. Four analytical platforms were employed to study the profiles of polar metabolites in mouse feces collected from adult BWF1 mice, and a total of 435 metabolites was identified. Of these, the abundance levels of 72 metabolites were significantly different between castrated and intact male groups, and 63 metabolites were different between female and male groups. Pathway analysis indicated that the pathway differences between castrated and intact male mice closely resembled the pathway differences between female and intact male mice, suggesting that low levels of androgens, whether due to depletion (castrated male) or endogenous (female), are associated with multiple fecal metabolomic alterations, which could potentially affect SLE progression. Our findings demonstrate that analyzing fecal metabolites using multiple analytical platforms holds great promise for detecting metabolomic alterations in complex disease model systems.
中文翻译:

在系统性红斑狼疮小鼠模型中,使用多个分析平台调查排便代谢产物的雄激素耗竭作用。
系统性红斑狼疮(SLE)是一种自身免疫性疾病,其特征是循环的自身抗体沉积在靶器官(例如肾脏)中,导致慢性炎症并最终破坏器官。在人类和狼疮的自发小鼠模型(例如NZBxNZW F1(BWF1)小鼠)中,雌性SLE在雌性中的发生率均远高于雄性。通过去势消耗雄激素会大大增加BWF1男性对狼疮的敏感性。我们比较了cast割的BWF1(雄激素耗竭)雄性,完整(雄激素耗竭)雄性和雌性小鼠的粪便代谢产物谱。四个分析平台用于研究从成年BWF1小鼠收集的小鼠粪便中极性代谢产物的分布,共鉴定出435种代谢产物。这些,去势和完整雄性之间72种代谢产物的丰度水平存在显着差异,而雌雄之间则有63种代谢产物存在差异。路径分析表明,cast割的和完整的雄性小鼠之间的路径差异与雌性和完整的雄性小鼠之间的路径差异非常相似,这表明低水平的雄激素(无论是由于耗竭(cast割的雄性)还是内源性(雌性)引起的)与多种因素有关。粪便代谢组学改变,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。路径分析表明,cast割的和完整的雄性小鼠之间的路径差异与雌性和完整的雄性小鼠之间的路径差异非常相似,这表明低水平的雄激素(无论是由于耗竭(cast割的雄性)还是内源性(雌性)引起的)与多种因素有关。粪便代谢组学改变,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。路径分析表明,cast割的和完整的雄性小鼠之间的路径差异与雌性和完整的雄性小鼠之间的路径差异非常相似,这表明低水平的雄激素(无论是由于耗竭(cast割的雄性)还是内源性(雌性)引起的)与多种因素有关。粪便代谢组学改变,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。与多种粪便代谢组学改变相关,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。与多种粪便代谢组学改变相关,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。
更新日期:2019-12-29
中文翻译:

在系统性红斑狼疮小鼠模型中,使用多个分析平台调查排便代谢产物的雄激素耗竭作用。
系统性红斑狼疮(SLE)是一种自身免疫性疾病,其特征是循环的自身抗体沉积在靶器官(例如肾脏)中,导致慢性炎症并最终破坏器官。在人类和狼疮的自发小鼠模型(例如NZBxNZW F1(BWF1)小鼠)中,雌性SLE在雌性中的发生率均远高于雄性。通过去势消耗雄激素会大大增加BWF1男性对狼疮的敏感性。我们比较了cast割的BWF1(雄激素耗竭)雄性,完整(雄激素耗竭)雄性和雌性小鼠的粪便代谢产物谱。四个分析平台用于研究从成年BWF1小鼠收集的小鼠粪便中极性代谢产物的分布,共鉴定出435种代谢产物。这些,去势和完整雄性之间72种代谢产物的丰度水平存在显着差异,而雌雄之间则有63种代谢产物存在差异。路径分析表明,cast割的和完整的雄性小鼠之间的路径差异与雌性和完整的雄性小鼠之间的路径差异非常相似,这表明低水平的雄激素(无论是由于耗竭(cast割的雄性)还是内源性(雌性)引起的)与多种因素有关。粪便代谢组学改变,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。路径分析表明,cast割的和完整的雄性小鼠之间的路径差异与雌性和完整的雄性小鼠之间的路径差异非常相似,这表明低水平的雄激素(无论是由于耗竭(cast割的雄性)还是内源性(雌性)引起的)与多种因素有关。粪便代谢组学改变,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。路径分析表明,cast割的和完整的雄性小鼠之间的路径差异与雌性和完整的雄性小鼠之间的路径差异非常相似,这表明低水平的雄激素(无论是由于耗竭(cast割的雄性)还是内源性(雌性)引起的)与多种因素有关。粪便代谢组学改变,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。与多种粪便代谢组学改变相关,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。与多种粪便代谢组学改变相关,可能会影响SLE进展。我们的发现表明,使用多种分析平台分析粪便代谢物对于检测复杂疾病模型系统中的代谢组学改变具有广阔的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号