当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Novel Lipidomics-Based Approach to Evaluating the Risk of Clinical Hepatotoxicity Potential of Drugs in 3D Human Microtissues.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.chemrestox.9b00364 Laura Goracci 1 , Aurora Valeri 2 , Simone Sciabola 3 , Michael D Aleo 4 , Wolfgang Moritz 5 , Jan Lichtenberg 5 , Gabriele Cruciani 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.chemrestox.9b00364 Laura Goracci 1 , Aurora Valeri 2 , Simone Sciabola 3 , Michael D Aleo 4 , Wolfgang Moritz 5 , Jan Lichtenberg 5 , Gabriele Cruciani 1
Affiliation
|
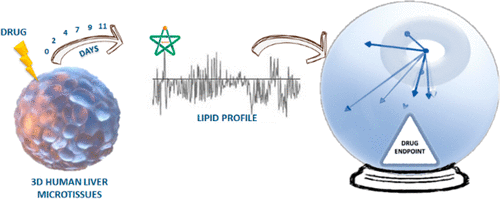
The importance of adsorption, distribution, metabolism, excretion, and toxicity (ADMET) analysis is expected to grow substantially due to recent failures in detecting severe toxicity issues of new chemical entities during preclinical/clinical development. Traditionally, safety risk assessment studies for humans have been conducted in animals during advanced preclinical or clinical phase of drug development. However, potential drug toxicity in humans now needs to be detected in the drug discovery process as soon as possible without reliance on animal studies. The "omics", such as genomics, proteomics, and metabolomics, have recently entered pharmaceutical research in both drug discovery and drug development, but to the best of our knowledge, no applications in high-throughput safety risk assessment have been attempted so far. This paper reports an innovative method to anticipate adverse drug effects in an early discovery phase based on lipid fingerprints using human three-dimensional microtissues. The risk of clinical hepatotoxicity potential was evaluated for a data set of 22 drugs belonging to five different therapeutic chemical classes and with various drug-induced liver injury effect. The treatment of microtissues with repeated doses of each drug allowed collecting lipid fingerprints for five time points (2, 4, 7, 9, and 11 days), and multivariate statistical analysis was applied to search for correlations with the hepatotoxic effect. The method allowed clustering of the drugs based on their hepatotoxic effect, and the observed lipid impairments for a number of drugs was confirmed by literature sources. Compared to traditional screening methods, here multiple interconnected variables (lipids) are measured simultaneously, providing a snapshot of the cellular status from the lipid perspective at a molecular level. Applied here to hepatotoxicity, the proposed workflow can be applied to several tissues, being tridimensional microtissues from various origins.
中文翻译:

一种新颖的基于脂质组学的方法来评估3D人体微组织中药物的临床肝毒性潜力的风险。
由于最近在临床前/临床开发过程中未能检测到新化学实体的严重毒性问题,因此吸附,分布,代谢,排泄和毒性(ADMET)分析的重要性预计将大大提高。传统上,人类的安全风险评估研究是在药物开发的高级临床前或临床阶段在动物中进行的。但是,现在需要在药物发现过程中尽快检测到对人类潜在的药物毒性,而无需依赖动物研究。基因组学,蛋白质组学和代谢组学等“组学”最近已进入药物研究和药物开发领域的药物研究,但是据我们所知,到目前为止,尚未尝试过在高通量安全风险评估中的应用。本文报告了一种创新的方法,该方法可以在使用人类三维微组织的脂质指纹的基础上,在早期发现阶段预测药物的不良作用。针对22种药物的数据集评估了临床肝毒性潜在风险,这些药物属于5种不同的化学治疗类别,并具有多种药物诱发的肝损伤作用。用重复剂量的每种药物治疗微组织可以收集五个时间点(2、4、7、9和11天)的脂质指纹,并应用多元统计分析来寻找与肝毒性作用的相关性。该方法基于其肝毒性作用使药物聚类,并且从文献资料中证实了观察到的许多药物的脂质损伤。与传统的筛查方法相比,在这里,同时测量了多个相互关联的变量(脂质),从脂质角度在分子水平上提供了细胞状态的快照。此处将其应用于肝毒性,可以将所提出的工作流程应用于多种组织,这些组织是来自各种起源的三维微组织。
更新日期:2019-12-25
中文翻译:

一种新颖的基于脂质组学的方法来评估3D人体微组织中药物的临床肝毒性潜力的风险。
由于最近在临床前/临床开发过程中未能检测到新化学实体的严重毒性问题,因此吸附,分布,代谢,排泄和毒性(ADMET)分析的重要性预计将大大提高。传统上,人类的安全风险评估研究是在药物开发的高级临床前或临床阶段在动物中进行的。但是,现在需要在药物发现过程中尽快检测到对人类潜在的药物毒性,而无需依赖动物研究。基因组学,蛋白质组学和代谢组学等“组学”最近已进入药物研究和药物开发领域的药物研究,但是据我们所知,到目前为止,尚未尝试过在高通量安全风险评估中的应用。本文报告了一种创新的方法,该方法可以在使用人类三维微组织的脂质指纹的基础上,在早期发现阶段预测药物的不良作用。针对22种药物的数据集评估了临床肝毒性潜在风险,这些药物属于5种不同的化学治疗类别,并具有多种药物诱发的肝损伤作用。用重复剂量的每种药物治疗微组织可以收集五个时间点(2、4、7、9和11天)的脂质指纹,并应用多元统计分析来寻找与肝毒性作用的相关性。该方法基于其肝毒性作用使药物聚类,并且从文献资料中证实了观察到的许多药物的脂质损伤。与传统的筛查方法相比,在这里,同时测量了多个相互关联的变量(脂质),从脂质角度在分子水平上提供了细胞状态的快照。此处将其应用于肝毒性,可以将所提出的工作流程应用于多种组织,这些组织是来自各种起源的三维微组织。











































 京公网安备 11010802027423号
京公网安备 11010802027423号