当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metabolite Profiling and Reaction Phenotyping for the in Vitro Assessment of the Bioactivation of Bromfenac †.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.chemrestox.9b00268 Aprajita S Yadav 1 , Nina R Shah 1 , Timothy J Carlson 1 , James P Driscoll 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.chemrestox.9b00268 Aprajita S Yadav 1 , Nina R Shah 1 , Timothy J Carlson 1 , James P Driscoll 1
Affiliation
|
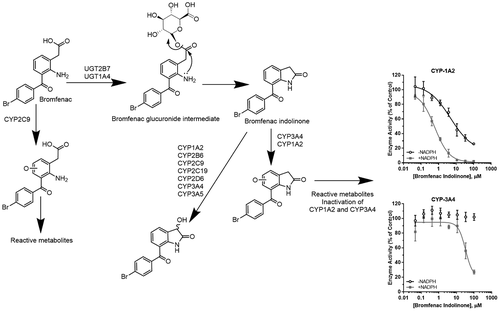
Bromfenac is a nonsteroidal anti-inflammatory drug that was approved and subsequently withdrawn from the market because of reported cases of acute hepatotoxicity. Recently, in vitro studies have revealed that bromfenac requires UDPGA and alamethicin supplemented human liver microsomes (HLM) to form a major metabolite, bromfenac indolinone (BI). Bromfenac and BI form thioether adducts through a bioactivation pathway in HLM and hepatocytes. [J. P. Driscoll et al., Chem. Res. Toxicol. 2018, 31, 223-230.] Here, Cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) reaction phenotyping experiments using recombinant enzymes were performed on bromfenac and BI to identify the CYP and UGT enzymes responsible for bromfenac's metabolism and bioactivation. It was determined that UGT2B7 converts bromfenac to BI, and that while CYP2C8, CYP2C9, and CYP2C19 catalyze the hydroxylation of bromfenac, only CYP2C9 forms thioether adducts when incubated with NAC or GSH as trapping agents. Although CYP2C9 was shown to form a reactive intermediate, no inhibition of CYP2C9 was observed when an IC50 shift assay was performed. Reaction phenotyping experiments with BI and recombinant CYP enzymes indicated that CYPs 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4 were responsible for the formation of an aliphatic hydroxylated metabolite. An aromatic hydroxylation on the indolinone moiety was also formed by CYP1A2 and CYP3A4. The aromatic hydroxylated BI is a precursor to the quinone methide and quinone imine intermediates in the proposed bioactivation pathway. Through time-dependent inhibition (TDI) experiments, it was revealed that BI can cause an IC50 shift in CYP1A2 and CYP3A4. However, BI does not inhibit the other isoforms that were also responsible for the formation of the aliphatic hydroxylation, an alternative biotransformation that does not undergo further downstream bioactivation. The results of these metabolism studies with bromfenac and BI add to our understanding of the relationship between biotransformation, reactive intermediate generation, and a potential mechanistic link to the hepatotoxicity of this compound.
中文翻译:

代谢物分析和反应表型用于体外评估溴芬酸†的生物活化作用。
溴芬酸是一种非甾体类抗炎药,由于报道了急性肝毒性病例,因此被批准并随后退出市场。近来,体外研究表明,溴芬酸需要UDPGA和补充有alamethicin的人肝微粒体(HLM)才能形成主要代谢物溴芬酸吲哚满酮(BI)。溴芬酸和BI通过HLM和肝细胞中的生物激活途径形成硫醚加合物。[JP Driscoll et al。,Chem。Res。毒药。2018,31,223-230。]在这里,对溴芬酸和BI进行了使用重组酶的细胞色素P450(CYP)和UDP-葡萄糖醛糖基转移酶(UGT)反应表型实验,以鉴定负责溴芬酸代谢和生物活化的CYP和UGT酶。已确定UGT2B7将溴芬酸转化为BI,而CYP2C8,CYP2C9,CYP2C19和CYP2C19催化溴芬酸的羟基化,只有当CYP2C9与NAC或GSH一起作为捕集剂孵育时,才会形成硫醚加合物。尽管显示CYP2C9形成反应性中间体,但进行IC50位移分析时未观察到CYP2C9的抑制作用。BI和重组CYP酶的反应表型实验表明,CYP 1A2、2B6、2C8、2C9、2C19、2D6和3A4负责脂肪族羟基化代谢产物的形成。CYP1A2和CYP3A4还在吲哚啉酮部分形成芳族羟基。芳香族羟基化的BI是拟议的生物活化途径中醌甲基化物和醌亚胺中间体的前体。通过时间依赖性抑制(TDI)实验,发现BI可以引起CYP1A2和CYP3A4的IC50改变。然而,BI不抑制也负责脂族羟基化的形成的其他同工型,其是不经历进一步下游生物活化的替代性生物转化。这些用溴芬酸和BI进行的代谢研究的结果加深了我们对生物转化,反应性中间产物以及与该化合物肝毒性的潜在机制联系之间关系的理解。
更新日期:2019-12-23
中文翻译:

代谢物分析和反应表型用于体外评估溴芬酸†的生物活化作用。
溴芬酸是一种非甾体类抗炎药,由于报道了急性肝毒性病例,因此被批准并随后退出市场。近来,体外研究表明,溴芬酸需要UDPGA和补充有alamethicin的人肝微粒体(HLM)才能形成主要代谢物溴芬酸吲哚满酮(BI)。溴芬酸和BI通过HLM和肝细胞中的生物激活途径形成硫醚加合物。[JP Driscoll et al。,Chem。Res。毒药。2018,31,223-230。]在这里,对溴芬酸和BI进行了使用重组酶的细胞色素P450(CYP)和UDP-葡萄糖醛糖基转移酶(UGT)反应表型实验,以鉴定负责溴芬酸代谢和生物活化的CYP和UGT酶。已确定UGT2B7将溴芬酸转化为BI,而CYP2C8,CYP2C9,CYP2C19和CYP2C19催化溴芬酸的羟基化,只有当CYP2C9与NAC或GSH一起作为捕集剂孵育时,才会形成硫醚加合物。尽管显示CYP2C9形成反应性中间体,但进行IC50位移分析时未观察到CYP2C9的抑制作用。BI和重组CYP酶的反应表型实验表明,CYP 1A2、2B6、2C8、2C9、2C19、2D6和3A4负责脂肪族羟基化代谢产物的形成。CYP1A2和CYP3A4还在吲哚啉酮部分形成芳族羟基。芳香族羟基化的BI是拟议的生物活化途径中醌甲基化物和醌亚胺中间体的前体。通过时间依赖性抑制(TDI)实验,发现BI可以引起CYP1A2和CYP3A4的IC50改变。然而,BI不抑制也负责脂族羟基化的形成的其他同工型,其是不经历进一步下游生物活化的替代性生物转化。这些用溴芬酸和BI进行的代谢研究的结果加深了我们对生物转化,反应性中间产物以及与该化合物肝毒性的潜在机制联系之间关系的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号