Chem ( IF 19.1 ) Pub Date : 2019-12-09 , DOI: 10.1016/j.chempr.2019.11.006 Satoshi Tominaka , Ryota Ishibiki , Asahi Fujino , Kohsaku Kawakami , Koji Ohara , Takuya Masuda , Iwao Matsuda , Hideo Hosono , Takahiro Kondo
|
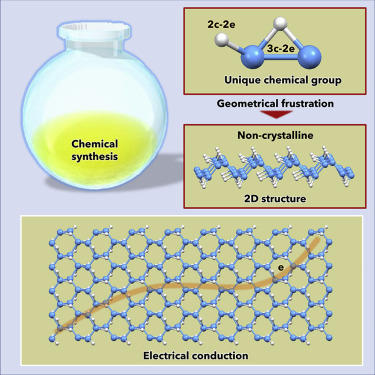
Hydrogen borides adopt a variety of structures because of the electron-deficient character of boron. Recently, we reported the synthesis of a layered hydrogen boride via a soft chemical route. Here, we ascertain the atomic arrangements in the layered hydrogen boride by using pair-distribution functions: the material dominantly consists of a corrugated B network decorated with three-center, two-electron B-H-B bridging bonds as well as ordinary two-center, two-electron B-H terminal bonds. The material is locally ordered but amorphous by diffractometry. This discrepancy can be accounted for by geometrical frustration caused by the positions of terminal B-H bonds located on one of two equivalent B atoms in the B-H-B bridging bonds. This material is electrically conductive (0.13 S cm−1 below 10°C) rather than ion conductive, and its B-H-B bonds are cleaved by the adsorption of molecules; this dynamic chemical nature originates from the frustrated structure and leads to unique hydrogen boride functionalities.
中文翻译:

软化学可及的层状氢硼化物中BH键的几何受阻
由于硼的电子缺乏特性,硼化氢采用多种结构。最近,我们报道了通过软化学路线合成层状硼化氢。在这里,我们通过成对分布函数确定层状硼化氢中的原子排列:该材料主要由波纹B网络组成,该B网络装饰有三中心,两个电子的BHB桥键以及普通的两个中心,两个-电子BH末端键。该材料是局部有序的,但是通过衍射法是非晶的。这种差异可以由位于BHB桥键中两个等效B原子之一上的末端BH键的位置引起的几何结构挫折来解决。该材料是导电的(0.13 S cm -1低于10°C)而不是离子导电性,并且其BHB键通过分子吸附被裂解; 这种动态化学性质源自受挫的结构,并导致独特的硼化氢功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号