当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-02-01 , DOI: 10.1016/s1474-4422(19)30395-3 Xinfeng Liu 1 , Qiliang Dai 2 , Ruidong Ye 2 , Wenjie Zi 3 , Yuxiu Liu 4 , Huaiming Wang 2 , Wusheng Zhu 2 , Minmin Ma 2 , Qin Yin 2 , Min Li 2 , Xinying Fan 2 , Wen Sun 2 , Yunfei Han 2 , Qiushi Lv 2 , Rui Liu 2 , Dong Yang 2 , Zhonghua Shi 5 , Dequan Zheng 6 , Xiaorong Deng 7 , Yue Wan 7 , Zhen Wang 8 , Yu Geng 9 , Xingyu Chen 10 , Zhiming Zhou 11 , Geng Liao 12 , Ping Jin 13 , Yumin Liu 14 , Xintong Liu 15 , Meng Zhang 16 , Feng Zhou 17 , Hongchao Shi 17 , Yunfeng Zhang 18 , Fuqiang Guo 19 , Congguo Yin 20 , Guozhong Niu 20 , Mei Zhang 21 , Xueli Cai 22 , Qiyi Zhu 23 , Zhonglun Chen 24 , Yingchun Liang 25 , Bing Li 26 , Min Lin 27 , Wei Wang 28 , Haowen Xu 29 , Xinmin Fu 30 , Wenhua Liu 31 , Xiguang Tian 32 , Zili Gong 33 , Haicun Shi 34 , Chuanming Wang 35 , Penghua Lv 36 , Zhonghai Tao 37 , Liangfu Zhu 38 , Shiquan Yang 39 , Wei Hu 40 , Pingzhou Jiang 41 , David S Liebeskind 42 , Vitor M Pereira 43 , Thomas Leung 44 , Bernard Yan 45 , Stephen Davis 45 , Gelin Xu 2 , Raul G Nogueira 46 ,
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-02-01 , DOI: 10.1016/s1474-4422(19)30395-3 Xinfeng Liu 1 , Qiliang Dai 2 , Ruidong Ye 2 , Wenjie Zi 3 , Yuxiu Liu 4 , Huaiming Wang 2 , Wusheng Zhu 2 , Minmin Ma 2 , Qin Yin 2 , Min Li 2 , Xinying Fan 2 , Wen Sun 2 , Yunfei Han 2 , Qiushi Lv 2 , Rui Liu 2 , Dong Yang 2 , Zhonghua Shi 5 , Dequan Zheng 6 , Xiaorong Deng 7 , Yue Wan 7 , Zhen Wang 8 , Yu Geng 9 , Xingyu Chen 10 , Zhiming Zhou 11 , Geng Liao 12 , Ping Jin 13 , Yumin Liu 14 , Xintong Liu 15 , Meng Zhang 16 , Feng Zhou 17 , Hongchao Shi 17 , Yunfeng Zhang 18 , Fuqiang Guo 19 , Congguo Yin 20 , Guozhong Niu 20 , Mei Zhang 21 , Xueli Cai 22 , Qiyi Zhu 23 , Zhonglun Chen 24 , Yingchun Liang 25 , Bing Li 26 , Min Lin 27 , Wei Wang 28 , Haowen Xu 29 , Xinmin Fu 30 , Wenhua Liu 31 , Xiguang Tian 32 , Zili Gong 33 , Haicun Shi 34 , Chuanming Wang 35 , Penghua Lv 36 , Zhonghai Tao 37 , Liangfu Zhu 38 , Shiquan Yang 39 , Wei Hu 40 , Pingzhou Jiang 41 , David S Liebeskind 42 , Vitor M Pereira 43 , Thomas Leung 44 , Bernard Yan 45 , Stephen Davis 45 , Gelin Xu 2 , Raul G Nogueira 46 ,
Affiliation
|
BACKGROUND
Previous randomised trials have shown an overwhelming benefit of mechanical thrombectomy for treating patients with stroke caused by large vessel occlusion of the anterior circulation. Whether endovascular treatment is beneficial for vertebrobasilar artery occlusion remains unknown. In this study, we aimed to investigate the safety and efficacy of endovascular treatment of acute strokes due to vertebrobasilar artery occlusion. METHODS
We did a multicentre, randomised, open-label trial, with blinded outcome assessment of thrombectomy in patients presenting within 8 h of vertebrobasilar occlusion at 28 centres in China. Patients were randomly assigned (1:1) to endovascular therapy plus standard medical therapy (intervention group) or standard medical therapy alone (control group). The randomisation sequence was computer-generated and stratified by participating centres. Allocation concealment was implemented by use of sealed envelopes. The primary outcome was a modified Rankin scale (mRS) score of 3 or lower (indicating ability to walk unassisted) at 90 days, assessed on an intention-to-treat basis. The primary safety outcome was mortality at 90 days. Secondary safety endpoints included the rates of symptomatic intracranial haemorrhage, device-related complications, and other severe adverse events. The BEST trial is registered with ClinicalTrials.gov, NCT02441556. FINDINGS
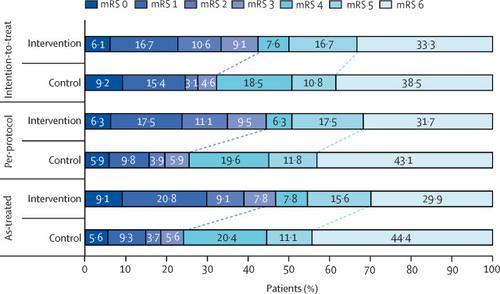
Between April 27, 2015, and Sept 27, 2017, we assessed 288 patients for eligibility. The trial was terminated early after 131 patients had been randomly assigned (66 patients to the intervention group and 65 to the control group) because of high crossover rate and poor recruitment. In the intention-to-treat analysis, there was no evidence of a difference in the proportion of participants with mRS 0-3 at 90 days according to treatment (28 [42%] of 66 patients in the intervention group vs 21 [32%] of 65 in the control group; adjusted odds ratio [OR] 1·74, 95% CI 0·81-3·74). Secondary prespecified analyses of the primary outcome, done to assess the effect of crossovers, showed higher rates of mRS 0-3 at 90 days in patients who actually received the intervention compared with those who received standard medical therapy alone in both per-protocol (28 [44%] of 63 patients with intervention vs 13 [25%] of 51 with standard therapy; adjusted OR 2·90, 95% CI 1·20-7·03) and as-treated (36 [47%] of 77 patients with intervention vs 13 [24%] of 54 with standard therapy; 3·02, 1·31-7·00) populations. The 90-day mortality was similar between groups (22 [33%] of 66 patients in the intervention vs 25 [38%] of 65 in the control group; p=0·54) despite a numerically higher prevalence of symptomatic intracranial haemorrhage in the intervention group. INTERPRETATION
There was no evidence of a difference in favourable outcomes of patients receiving endovascular therapy compared with those receiving standard medical therapy alone. Results might have been confounded by loss of equipoise over the course of the trial, resulting in poor adherence to the assigned study treatment and a reduced sample size due to the early termination of the study. FUNDING
Jiangsu Provincial Special Program of Medical Science.
更新日期:2020-02-01











































 京公网安备 11010802027423号
京公网安备 11010802027423号