Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-12-10 , DOI: 10.1016/s0140-6736(19)32956-3 Maria-Victoria Mateos 1 , Michele Cavo 2 , Joan Blade 3 , Meletios A Dimopoulos 4 , Kenshi Suzuki 5 , Andrzej Jakubowiak 6 , Stefan Knop 7 , Chantal Doyen 8 , Paulo Lucio 9 , Zsolt Nagy 10 , Ludek Pour 11 , Mark Cook 12 , Sebastian Grosicki 13 , Andre Crepaldi 14 , Anna Marina Liberati 15 , Philip Campbell 16 , Tatiana Shelekhova 17 , Sung-Soo Yoon 18 , Genadi Iosava 19 , Tomoaki Fujisaki 20 , Mamta Garg 21 , Maria Krevvata 22 , Ying Chen 23 , Jianping Wang 23 , Anupa Kudva 23 , Jon Ukropec 24 , Susan Wroblewski 22 , Ming Qi 22 , Rachel Kobos 23 , Jesus San-Miguel 25
The Lancet ( IF 98.4 ) Pub Date : 2019-12-10 , DOI: 10.1016/s0140-6736(19)32956-3 Maria-Victoria Mateos 1 , Michele Cavo 2 , Joan Blade 3 , Meletios A Dimopoulos 4 , Kenshi Suzuki 5 , Andrzej Jakubowiak 6 , Stefan Knop 7 , Chantal Doyen 8 , Paulo Lucio 9 , Zsolt Nagy 10 , Ludek Pour 11 , Mark Cook 12 , Sebastian Grosicki 13 , Andre Crepaldi 14 , Anna Marina Liberati 15 , Philip Campbell 16 , Tatiana Shelekhova 17 , Sung-Soo Yoon 18 , Genadi Iosava 19 , Tomoaki Fujisaki 20 , Mamta Garg 21 , Maria Krevvata 22 , Ying Chen 23 , Jianping Wang 23 , Anupa Kudva 23 , Jon Ukropec 24 , Susan Wroblewski 22 , Ming Qi 22 , Rachel Kobos 23 , Jesus San-Miguel 25
Affiliation
|
BACKGROUND
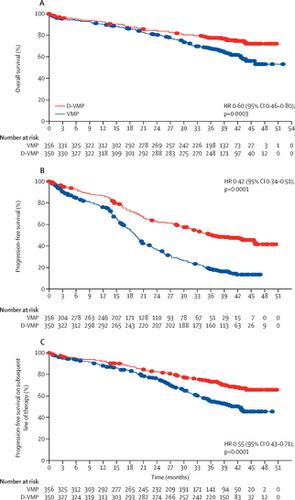
Standard-of-care treatment for patients with newly diagnosed multiple myeloma includes combination therapies for patients who are not eligible for autologous stem-cell transplantation. At the primary analysis for progression-free survival of the phase 3 ALCYONE trial, progression-free survival was significantly longer with daratumumab in combination with bortezomib, melphalan, and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone (VMP) alone in patients with transplant-ineligible, newly diagnosed multiple myeloma. Here we report updated efficacy and safety results from a prespecified, interim, overall survival analysis of ALCYONE with more than 36 months of follow-up.
METHODS
ALCYONE was a multicentre, randomised, open-label, active-controlled, phase 3 trial that enrolled patients between Feb 9, 2015, and July 14, 2016, at 162 sites in 25 countries across North America, South America, Europe, and the Asia-Pacific region. Patients were eligible for inclusion if they had newly diagnosed multiple myeloma and were ineligible for high-dose chemotherapy with autologous stem-cell transplantation, because of their age (≥65 years) or because of substantial comorbidities. Patients were randomly assigned in a 1:1 ratio and by permuted block randomisation to receive D-VMP or VMP. An interactive web-based randomisation system was used. Randomisation was stratified by International Staging System disease stage, geographical region, and age. There was no masking to treatment assignments. All patients received up to nine 6-week cycles of subcutaneous bortezomib (1·3 mg/m2 of body surface area on days 1, 4, 8, 11, 22, 25, 29, and 32 of cycle one and on days 1, 8, 22, and 29 of cycles two through nine), oral melphalan (9 mg/m2 once daily on days 1 through 4 of each cycle), and oral prednisone (60 mg/m2 once daily on days 1 through 4 of each cycle). Patients in the D-VMP group also received intravenous daratumumab (16 mg/kg of bodyweight, once weekly during cycle one, once every 3 weeks in cycles two through nine, and once every 4 weeks thereafter as maintenance therapy until disease progression or unacceptable toxicity). The primary endpoint was progression-free survival, which has been reported previously. Results presented are from a prespecified interim analysis for overall survival. The primary analysis population (including for overall survival) was the intention-to-treat population of all patients who were randomly assigned to treatment. The safety population included patients who received any dose of study treatment. This trial is registered with ClinicalTrials.gov, NCT02195479.
FINDINGS
706 patients were randomly assigned to treatment groups (350 to the D-VMP group, 356 to the VMP group). At a median follow-up of 40·1 months (IQR 37·4-43·1), a significant benefit in overall survival was observed for the D-VMP group. The hazard ratio (HR) for death in the D-VMP group compared with the VMP group was 0·60 (95% CI 0·46-0·80; p=0·0003). The Kaplan-Meier estimate of the 36-month rate of overall survival was 78·0% (95% CI 73·2-82·0) in the D-VMP group and 67·9% (62·6-72·6) in the VMP group. Progression-free survival, the primary endpoint, remained significantly improved for the D-VMP group (HR 0·42 [0·34-0·51]; p<0·0001). The most frequent adverse events during maintenance daratumumab monotherapy in patients in the D-VMP group were respiratory infections (54 [19%] of 278 patients had upper respiratory tract infections; 42 [15%] had bronchitis, 34 [12%] had viral upper respiratory tract infections), cough (34 [12%]), and diarrhoea (28 [10%]).
INTERPRETATION
D-VMP prolonged overall survival in patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation. With more than 3 years of follow-up, the D-VMP group continued to show significant improvement in progression-free survival, with no new safety concerns.
FUNDING
Janssen Research & Development.
中文翻译:

新诊断的多发性骨髓瘤(ALCYONE)中使用daratumumab,bortezomib,melphalan和泼尼松的总生存期:一项随机,开放标签的3期试验。
背景技术对于新诊断为多发性骨髓瘤的患者的护理标准治疗包括针对不适合自体干细胞移植的患者的联合疗法。在3期ALCYONE试验的无进展生存期的初步分析中,与单独使用硼替佐米,美法仑和泼尼松(VMP)相比,daratumumab联合硼替佐米,美法仑和泼尼松(D-VMP)的无进展生存期明显更长不适合移植的,新诊断的多发性骨髓瘤患者。在这里,我们报告了对ALCYONE进行的预定,中期总体生存分析的最新疗效和安全性结果,并进行了36个月以上的随访。方法ALCYONE是一项多中心,随机,开放标签,主动控制的3期临床试验,纳入了2015年2月9日至2016年7月14日之间的患者,遍布北美,南美,欧洲和亚太地区25个国家/地区的162个站点。如果患者是新诊断的多发性骨髓瘤,并且由于年龄(≥65岁)或严重合并症而不能进行自体干细胞移植的大剂量化疗,则符合入选条件。患者按1:1比例随机分配,并按排列的区组随机分组接受D-VMP或VMP。使用了基于网络的交互式随机系统。根据国际分期系统疾病的阶段,地理区域和年龄对随机分组进行分层。没有掩盖治疗分配。所有患者在第1周期的第1、4、8、11、22、25、29和32天以及第1天,都接受了多达9个6周的硼替佐米皮下周期治疗(体表面积的1·3 mg / m2)。 8、22,和第2至9个周期的29个),口服美法仑(每个周期的第1至4天每天一次9 mg / m2)和口服泼尼松(每个周期的第1至4天每天一次60 mg / m2)。D-VMP组的患者还接受静脉注射daratumumab(16 mg / kg体重,在第一个周期内每周一次,在第二个至第九个周期内每3周一次,然后每4周一次作为维持治疗,直到疾病进展或出现不可接受的毒性)。主要终点是无进展生存期,先前已有报道。给出的结果来自预先确定的总体生存期中期分析。主要分析人群(包括总体生存期)为所有随机分配接受治疗的患者的意向治疗人群。安全人群包括接受任何剂量研究治疗的患者。该试验已在ClinicalTrials.gov上注册,NCT02195479。结果将706例患者随机分为治疗组(D-VMP组350例,VMP组356例)。在中位随访40·1个月(IQR 37·4-43·1)时,D-VMP组在总体生存率方面有显着获益。与VMP组相比,D-VMP组死亡的危险比(HR)为0·60(95%CI 0·46-0·80; p = 0·0003)。Kaplan-Meier评估的D-VMP组36个月总生存率为78·0%(95%CI 73·2-82·0)和67·9%(62·6-72·6) )。D-VMP组的主要终点无进展生存率仍得到显着改善(HR 0·42 [0·34-0·51]; p <0·0001)。D-VMP组患者在维持daratumumab单药治疗期间最常见的不良事件是呼吸道感染(278例患者中有54例[19%]患有上呼吸道感染; 42例[15%]患有支气管炎,34例[12%]患有病毒性感染)上呼吸道感染),咳嗽(34 [12%])和腹泻(28 [10%])。解释D-VMP延长了新诊断为不适合干细胞移植的多发性骨髓瘤患者的总体生存期。经过3年多的随访,D-VMP组继续表现出无进展生存期的显着改善,而无新的安全隐患。资金Janssen研究与开发。34 [12%]有病毒性上呼吸道感染),咳嗽(34 [12%])和腹泻(28 [10%])。解释D-VMP延长了新诊断为不适合干细胞移植的多发性骨髓瘤患者的总体生存期。经过3年多的随访,D-VMP组继续表现出无进展生存期的显着改善,而无新的安全隐患。资金Janssen研究与开发。34 [12%]有病毒性上呼吸道感染),咳嗽(34 [12%])和腹泻(28 [10%])。解释D-VMP延长了新诊断为不适合干细胞移植的多发性骨髓瘤患者的总体生存期。经过3年多的随访,D-VMP组继续表现出无进展生存期的显着改善,而无新的安全隐患。资金Janssen研究与开发。
更新日期:2020-01-10
中文翻译:

新诊断的多发性骨髓瘤(ALCYONE)中使用daratumumab,bortezomib,melphalan和泼尼松的总生存期:一项随机,开放标签的3期试验。
背景技术对于新诊断为多发性骨髓瘤的患者的护理标准治疗包括针对不适合自体干细胞移植的患者的联合疗法。在3期ALCYONE试验的无进展生存期的初步分析中,与单独使用硼替佐米,美法仑和泼尼松(VMP)相比,daratumumab联合硼替佐米,美法仑和泼尼松(D-VMP)的无进展生存期明显更长不适合移植的,新诊断的多发性骨髓瘤患者。在这里,我们报告了对ALCYONE进行的预定,中期总体生存分析的最新疗效和安全性结果,并进行了36个月以上的随访。方法ALCYONE是一项多中心,随机,开放标签,主动控制的3期临床试验,纳入了2015年2月9日至2016年7月14日之间的患者,遍布北美,南美,欧洲和亚太地区25个国家/地区的162个站点。如果患者是新诊断的多发性骨髓瘤,并且由于年龄(≥65岁)或严重合并症而不能进行自体干细胞移植的大剂量化疗,则符合入选条件。患者按1:1比例随机分配,并按排列的区组随机分组接受D-VMP或VMP。使用了基于网络的交互式随机系统。根据国际分期系统疾病的阶段,地理区域和年龄对随机分组进行分层。没有掩盖治疗分配。所有患者在第1周期的第1、4、8、11、22、25、29和32天以及第1天,都接受了多达9个6周的硼替佐米皮下周期治疗(体表面积的1·3 mg / m2)。 8、22,和第2至9个周期的29个),口服美法仑(每个周期的第1至4天每天一次9 mg / m2)和口服泼尼松(每个周期的第1至4天每天一次60 mg / m2)。D-VMP组的患者还接受静脉注射daratumumab(16 mg / kg体重,在第一个周期内每周一次,在第二个至第九个周期内每3周一次,然后每4周一次作为维持治疗,直到疾病进展或出现不可接受的毒性)。主要终点是无进展生存期,先前已有报道。给出的结果来自预先确定的总体生存期中期分析。主要分析人群(包括总体生存期)为所有随机分配接受治疗的患者的意向治疗人群。安全人群包括接受任何剂量研究治疗的患者。该试验已在ClinicalTrials.gov上注册,NCT02195479。结果将706例患者随机分为治疗组(D-VMP组350例,VMP组356例)。在中位随访40·1个月(IQR 37·4-43·1)时,D-VMP组在总体生存率方面有显着获益。与VMP组相比,D-VMP组死亡的危险比(HR)为0·60(95%CI 0·46-0·80; p = 0·0003)。Kaplan-Meier评估的D-VMP组36个月总生存率为78·0%(95%CI 73·2-82·0)和67·9%(62·6-72·6) )。D-VMP组的主要终点无进展生存率仍得到显着改善(HR 0·42 [0·34-0·51]; p <0·0001)。D-VMP组患者在维持daratumumab单药治疗期间最常见的不良事件是呼吸道感染(278例患者中有54例[19%]患有上呼吸道感染; 42例[15%]患有支气管炎,34例[12%]患有病毒性感染)上呼吸道感染),咳嗽(34 [12%])和腹泻(28 [10%])。解释D-VMP延长了新诊断为不适合干细胞移植的多发性骨髓瘤患者的总体生存期。经过3年多的随访,D-VMP组继续表现出无进展生存期的显着改善,而无新的安全隐患。资金Janssen研究与开发。34 [12%]有病毒性上呼吸道感染),咳嗽(34 [12%])和腹泻(28 [10%])。解释D-VMP延长了新诊断为不适合干细胞移植的多发性骨髓瘤患者的总体生存期。经过3年多的随访,D-VMP组继续表现出无进展生存期的显着改善,而无新的安全隐患。资金Janssen研究与开发。34 [12%]有病毒性上呼吸道感染),咳嗽(34 [12%])和腹泻(28 [10%])。解释D-VMP延长了新诊断为不适合干细胞移植的多发性骨髓瘤患者的总体生存期。经过3年多的随访,D-VMP组继续表现出无进展生存期的显着改善,而无新的安全隐患。资金Janssen研究与开发。







































 京公网安备 11010802027423号
京公网安备 11010802027423号