当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Flow Process Built upon a Batch Foundation—Preparation of a Key Amino Alcohol Intermediate via Multistage Continuous Synthesis
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-12-10 , DOI: 10.1021/acs.oprd.9b00478 John Jin Lim 1 , Kenneth Arrington 1 , Anna L. Dunn 1 , David C. Leitch 1, 2 , Ian Andrews 1 , Neil R. Curtis 3 , Mark J. Hughes 3 , Daniel R. Tray 3 , Charles E. Wade 3 , Matthew P. Whiting 3 , Charles Goss 4 , Yangmu Chloe Liu 3 , Brian M. Roesch 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-12-10 , DOI: 10.1021/acs.oprd.9b00478 John Jin Lim 1 , Kenneth Arrington 1 , Anna L. Dunn 1 , David C. Leitch 1, 2 , Ian Andrews 1 , Neil R. Curtis 3 , Mark J. Hughes 3 , Daniel R. Tray 3 , Charles E. Wade 3 , Matthew P. Whiting 3 , Charles Goss 4 , Yangmu Chloe Liu 3 , Brian M. Roesch 3
Affiliation
|
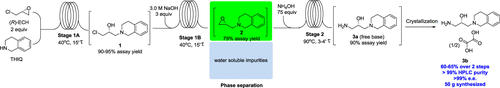
This paper describes recent efforts to apply flow technology in the preparation of the key amino alcohol intermediate 3b so as to address manufacturability issues present in the batch process of a PRMT5 inhibitor. The continuous process, one of the first reported pharmaceutical processes to use aqueous NH4OH in flow, eliminates an isolation and the use of dichloromethane in the workup and improves reaction time >140-fold compared with the batch process to deliver multigram quantities of 3b in 60–65% isolated yield with >99 HPLC area % and >99% ee. While the flow process greatly increases the efficiency compared with the batch process, small-scale batch experiments were crucial in gaining reaction understanding to increase the kinetics and minimize impurity formation. The holistic process design underscores our belief that large-scale flow processes are built upon the knowledge gained through well-chosen small-scale batch experiments.
中文翻译:

基于批处理基础的流程-通过多阶段连续合成制备关键的氨基醇中间体
本文介绍了在关键氨基醇中间体3b的制备中应用流动技术的最新成果,以解决PRMT5抑制剂分批工艺中存在的可制造性问题。连续过程是最早报道的使用NH 4 OH水溶液流动的制药过程之一,与分批过程相比,该方法无需分离和在处理过程中使用二氯甲烷,并且将反应时间提高了140倍以上,从而可交付数克3b的药物。分离出的收率为60–65%,HPLC面积> 99,ee≥99%。尽管与分批工艺相比,流动工艺大大提高了效率,但小规模的分批实验对于获得对反应的认识,以增加动力学并最大程度地减少杂质形成至关重要。整体过程设计强调了我们的信念,即大规模流程是建立在通过精心选择的小规模批量实验获得的知识之上的。
更新日期:2019-12-10
中文翻译:

基于批处理基础的流程-通过多阶段连续合成制备关键的氨基醇中间体
本文介绍了在关键氨基醇中间体3b的制备中应用流动技术的最新成果,以解决PRMT5抑制剂分批工艺中存在的可制造性问题。连续过程是最早报道的使用NH 4 OH水溶液流动的制药过程之一,与分批过程相比,该方法无需分离和在处理过程中使用二氯甲烷,并且将反应时间提高了140倍以上,从而可交付数克3b的药物。分离出的收率为60–65%,HPLC面积> 99,ee≥99%。尽管与分批工艺相比,流动工艺大大提高了效率,但小规模的分批实验对于获得对反应的认识,以增加动力学并最大程度地减少杂质形成至关重要。整体过程设计强调了我们的信念,即大规模流程是建立在通过精心选择的小规模批量实验获得的知识之上的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号