当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Why do thioureas and squaramides slow down the Ireland-Claisen rearrangement?
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-12-10 , DOI: 10.3762/bjoc.15.290 Dominika Krištofíková 1 , Juraj Filo 2 , Mária Mečiarová 1 , Radovan Šebesta 1
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-12-10 , DOI: 10.3762/bjoc.15.290 Dominika Krištofíková 1 , Juraj Filo 2 , Mária Mečiarová 1 , Radovan Šebesta 1
Affiliation
|
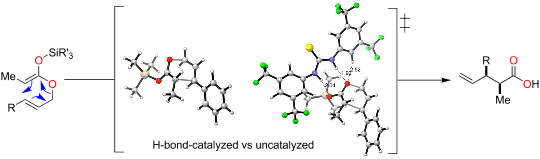
A range of chiral hydrogen-bond-donating organocatalysts was tested in the Ireland-Claisen rearrangement of silyl ketene acetals. None of these organocatalysts was able to impart any enantioselectivity on the rearrangements. Furthermore, these organocatalysts slowed down the Ireland-Claisen rearrangement in comparison to an uncatalyzed reaction. The catalyst-free reaction proceeded well in green solvents or without any solvent. DFT calculations showed that the activation barriers are higher for reactions involving hydrogen-donating organocatalysts and kinetic experiments suggest that the catalysts bind stronger to the starting silyl ketene acetals than to transition structures thus leading to inefficient rearrangement reactions.
中文翻译:

为什么硫脲和方胺会减缓爱尔兰-克莱森的重排?
在甲硅烷基乙烯酮缩醛的爱尔兰-克莱森重排中测试了一系列手性给氢键的有机催化剂。这些有机催化剂均不能在重排上赋予任何对映选择性。此外,与未催化反应相比,这些有机催化剂减缓了爱尔兰-克莱森重排。在绿色溶剂中或没有任何溶剂的情况下,无催化剂的反应进展顺利。DFT计算表明,涉及供氢有机催化剂的反应的活化能垒较高,动力学实验表明,催化剂与起始甲硅烷基乙烯酮缩醛的结合力强于过渡结构,从而导致重排反应效率低下。
更新日期:2019-12-11
中文翻译:

为什么硫脲和方胺会减缓爱尔兰-克莱森的重排?
在甲硅烷基乙烯酮缩醛的爱尔兰-克莱森重排中测试了一系列手性给氢键的有机催化剂。这些有机催化剂均不能在重排上赋予任何对映选择性。此外,与未催化反应相比,这些有机催化剂减缓了爱尔兰-克莱森重排。在绿色溶剂中或没有任何溶剂的情况下,无催化剂的反应进展顺利。DFT计算表明,涉及供氢有机催化剂的反应的活化能垒较高,动力学实验表明,催化剂与起始甲硅烷基乙烯酮缩醛的结合力强于过渡结构,从而导致重排反应效率低下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号