当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiophene bioisosteres of GluN2B selective NMDA receptor antagonists: Synthesis and pharmacological evaluation of [7]annuleno[b]thiophen-6-amines.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-09 , DOI: 10.1016/j.bmc.2019.115245 Sören Baumeister 1 , Dirk Schepmann 1 , Bernhard Wünsch 2
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-09 , DOI: 10.1016/j.bmc.2019.115245 Sören Baumeister 1 , Dirk Schepmann 1 , Bernhard Wünsch 2
Affiliation
|
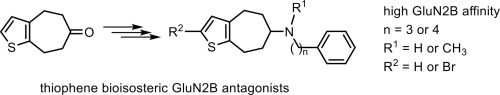
Thiophene bioisosteres of potent GluN2B receptor negative allosteric modulators were prepared and evaluated pharmacologically. The five-step synthesis of 4,5,7,8-tetrahydro[7]annuleno[b]thiophen-6-one (10) was considerably improved by carboxylation of thiophene-3-carboxylic acid (8) in the first reaction step. Reductive amination and alkylation led to three homologous series of secondary and tertiary phenylalkylamines 5, 11 and 12. Metalation, reaction with 1-formylpiperidine and subsequent reduction provided hydroxymethyl derivatives 15 and 16, which had been designed as bioisosteres of phenols. 2-Bromo derivatives 18 were obtained by bromination of ketone 10 with NBS and subsequent reductive amination. High GluN2B affinity was achieved with [7]annuleno[b]thiophenes bearing a 3-phenylpropylamino or 4-phenylbutylamino moiety (e.g. 5c: Ki = 5.9 nM; 11d: Ki = 9.0 nM). Tertiary ethylamines 12 showed lower GluN2B affinity than tertiary methylamines 11 or secondary amines 5 (e.g. 5c: Ki = 5.9 nM; 11c: Ki = 6.0; 12c: Ki = 51 nM). A Br-atom or a hydroxymethyl moiety in 2-position were less tolerated by the GluN2B receptor. Very similar relationships between the structure and GluN2B affinity and structure and σ affinity, in particular σ2 affinity, were detected. A slight preference for the ifenprodil binding site of GluN2B receptors over σ1 and σ2 receptors was found for methylamines 11c (≈2-fold) and 11d (≈1.5-2-fold) as well as for bromo derivative 18c (≈3-fold).
中文翻译:

GluN2B选择性NMDA受体拮抗剂的噻吩类生物电子等排体:[7]环戊[b]噻吩-6-胺的合成和药理学评估。
制备了强效的GluN2B受体阴性变构调节剂的噻吩生物同工异构体,并进行了药理学评估。在第一步反应中,通过噻吩-3-羧酸(8)的羧化反应,大大改善了4,5,7,8-四氢[7]环核[b]噻吩-6-(10)的五步合成。还原胺化和烷基化产生了三个同源系列的仲和叔苯基烷基胺5、11和12。金属化,与1-甲酰基哌啶反应并随后还原得到了羟甲基衍生物15和16,它们被设计为苯酚的生物等排体。通过用NBS溴化酮10并随后进行还原性胺化反应,获得了2-溴衍生物18。使用带有3-苯基丙基氨基或4-苯基丁基氨基部分的[7]环戊[b]噻吩实现了高GluN2B亲和力(例如5c:Ki = 5.9 nM;11d:Ki = 9.0nM)。叔乙胺12显示出比叔甲胺11或仲胺5低的GluN2B亲和力(例如5c:Ki = 5.9 nM; 11c:Ki = 6.0; 12c:Ki = 51 nM)。GluN2B受体对2位的Br原子或羟甲基部分的耐受性较低。检测到结构和GluN2B亲和力与结构和σ亲和力,特别是σ2亲和力之间非常相似的关系。发现甲胺11c(≈2倍)和11d(≈1.5-2倍)以及溴衍生物18c(≈3倍)对GluN2B受体的ifenprodil结合位点的偏爱程度高于σ1和σ2受体。 。GluN2B受体对2位的Br原子或羟甲基部分的耐受性较低。检测到结构和GluN2B亲和力与结构和σ亲和力,特别是σ2亲和力之间非常相似的关系。发现甲胺11c(≈2倍)和11d(≈1.5-2倍)以及溴衍生物18c(≈3倍)对GluN2B受体的ifenprodil结合位点的偏爱程度高于σ1和σ2受体。 。GluN2B受体对2位的Br原子或羟甲基部分的耐受性较低。检测到结构和GluN2B亲和力与结构和σ亲和力,特别是σ2亲和力之间非常相似的关系。发现甲胺11c(≈2倍)和11d(≈1.5-2倍)以及溴衍生物18c(≈3倍)对GluN2B受体的ifenprodil结合位点的偏爱程度高于σ1和σ2受体。 。
更新日期:2019-12-09
中文翻译:

GluN2B选择性NMDA受体拮抗剂的噻吩类生物电子等排体:[7]环戊[b]噻吩-6-胺的合成和药理学评估。
制备了强效的GluN2B受体阴性变构调节剂的噻吩生物同工异构体,并进行了药理学评估。在第一步反应中,通过噻吩-3-羧酸(8)的羧化反应,大大改善了4,5,7,8-四氢[7]环核[b]噻吩-6-(10)的五步合成。还原胺化和烷基化产生了三个同源系列的仲和叔苯基烷基胺5、11和12。金属化,与1-甲酰基哌啶反应并随后还原得到了羟甲基衍生物15和16,它们被设计为苯酚的生物等排体。通过用NBS溴化酮10并随后进行还原性胺化反应,获得了2-溴衍生物18。使用带有3-苯基丙基氨基或4-苯基丁基氨基部分的[7]环戊[b]噻吩实现了高GluN2B亲和力(例如5c:Ki = 5.9 nM;11d:Ki = 9.0nM)。叔乙胺12显示出比叔甲胺11或仲胺5低的GluN2B亲和力(例如5c:Ki = 5.9 nM; 11c:Ki = 6.0; 12c:Ki = 51 nM)。GluN2B受体对2位的Br原子或羟甲基部分的耐受性较低。检测到结构和GluN2B亲和力与结构和σ亲和力,特别是σ2亲和力之间非常相似的关系。发现甲胺11c(≈2倍)和11d(≈1.5-2倍)以及溴衍生物18c(≈3倍)对GluN2B受体的ifenprodil结合位点的偏爱程度高于σ1和σ2受体。 。GluN2B受体对2位的Br原子或羟甲基部分的耐受性较低。检测到结构和GluN2B亲和力与结构和σ亲和力,特别是σ2亲和力之间非常相似的关系。发现甲胺11c(≈2倍)和11d(≈1.5-2倍)以及溴衍生物18c(≈3倍)对GluN2B受体的ifenprodil结合位点的偏爱程度高于σ1和σ2受体。 。GluN2B受体对2位的Br原子或羟甲基部分的耐受性较低。检测到结构和GluN2B亲和力与结构和σ亲和力,特别是σ2亲和力之间非常相似的关系。发现甲胺11c(≈2倍)和11d(≈1.5-2倍)以及溴衍生物18c(≈3倍)对GluN2B受体的ifenprodil结合位点的偏爱程度高于σ1和σ2受体。 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号