当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A molecular electron density theory study of the enhanced reactivity of aza aromatic compounds participating in Diels-Alder reactions.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-12-17 , DOI: 10.1039/c9ob02467k Luis R Domingo 1 , Mar Ríos-Gutiérrez , Patricia Pérez
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-12-17 , DOI: 10.1039/c9ob02467k Luis R Domingo 1 , Mar Ríos-Gutiérrez , Patricia Pérez
Affiliation
|
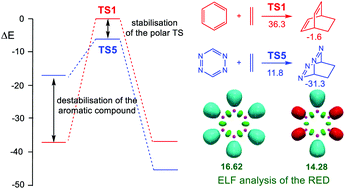
The enhanced reactivity of a series of four aza aromatic compounds (AACs) participating in the Diels-Alder (DA) reactions with ethylene has been studied using Molecular Electron Density Theory (MEDT). The analysis of the electronic structure of these AACs allows establishing that the substitution of the C-H unity by the isoelectronic N: unity linearly decreases the ring electron density (RED) of these compounds and concomitantly decreases their aromatic character and increases their electrophilic character. These behaviours not only decrease drastically the activation energies of these DA reactions, but also increase the reaction energies when they are compared with the very unfavourable DA reaction between benzene and ethylene. Very good correlations between the NICS(0) values and the electrophilicity ω indices of these AACs with the RED values are found. The present MEDT study makes it possible to establish two empirical electron density unity (EDU) indices accounting for the contribution of the C and N unities, 2.77 and 2.19 e, respectively, for the RED, which is mainly responsible for the reactivity of these AACs. Comprehensive chemical concepts such as electron density, aromaticity and electrophilicity make it possible to explain the chemical reactivity of these AACs participating in DA reactions towards ethylene.
中文翻译:

分子电子密度理论研究了参与Diels-Alder反应的氮杂芳族化合物的增强反应性。
已使用分子电子密度理论(MEDT)研究了参与Diels-Alder(DA)反应的一系列四个氮杂芳族化合物(AAC)与乙烯的增强反应性。通过对这些AAC的电子结构进行分析,可以确定用等电子N:CH取代CH线性可线性降低这些化合物的环电子密度(RED),并同时降低其芳族特性并增强其亲电子特性。与苯和乙烯之间非常不利的DA反应相比,这些行为不仅大大降低了这些DA反应的活化能,而且还增加了反应能。发现这些AAC的NICS(0)值和亲电性ω指数与RED值之间具有很好的相关性。目前的MEDT研究使建立两个经验电子密度单位(EDU)指数成为可能,这两个指标分别说明了C和N单位对RED的贡献,分别为2.77和2.19 e。 。全面的化学概念,例如电子密度,芳香性和亲电性,使得可以解释参与DA反应的这些AAC对乙烯的化学反应性。这主要负责这些AAC的反应性。诸如电子密度,芳香性和亲电性等全面的化学概念使我们有可能解释参与DA反应的这些AAC对乙烯的化学反应性。这主要负责这些AAC的反应性。全面的化学概念,例如电子密度,芳香性和亲电性,使得可以解释参与DA反应的这些AAC对乙烯的化学反应性。
更新日期:2020-01-15
中文翻译:

分子电子密度理论研究了参与Diels-Alder反应的氮杂芳族化合物的增强反应性。
已使用分子电子密度理论(MEDT)研究了参与Diels-Alder(DA)反应的一系列四个氮杂芳族化合物(AAC)与乙烯的增强反应性。通过对这些AAC的电子结构进行分析,可以确定用等电子N:CH取代CH线性可线性降低这些化合物的环电子密度(RED),并同时降低其芳族特性并增强其亲电子特性。与苯和乙烯之间非常不利的DA反应相比,这些行为不仅大大降低了这些DA反应的活化能,而且还增加了反应能。发现这些AAC的NICS(0)值和亲电性ω指数与RED值之间具有很好的相关性。目前的MEDT研究使建立两个经验电子密度单位(EDU)指数成为可能,这两个指标分别说明了C和N单位对RED的贡献,分别为2.77和2.19 e。 。全面的化学概念,例如电子密度,芳香性和亲电性,使得可以解释参与DA反应的这些AAC对乙烯的化学反应性。这主要负责这些AAC的反应性。诸如电子密度,芳香性和亲电性等全面的化学概念使我们有可能解释参与DA反应的这些AAC对乙烯的化学反应性。这主要负责这些AAC的反应性。全面的化学概念,例如电子密度,芳香性和亲电性,使得可以解释参与DA反应的这些AAC对乙烯的化学反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号