当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism and stereoselectivity of benzylic C-H hydroxylation by Ru-porphyrin: a computational study.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-12-17 , DOI: 10.1039/c9ob02415h Xiahe Chen 1 , Qunmin Wang , Haimin Shen , Guijie Li , Yun-Fang Yang , Yuan-Bin She
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-12-17 , DOI: 10.1039/c9ob02415h Xiahe Chen 1 , Qunmin Wang , Haimin Shen , Guijie Li , Yun-Fang Yang , Yuan-Bin She
Affiliation
|
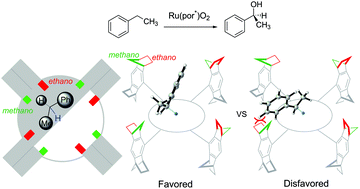
The mechanism and origin of the stereoselectivity of asymmetric benzylic C-H hydroxylation by Ru-porphyrin were elucidated with density functional theory calculations. The reaction proceeds via a hydrogen-atom abstraction/oxygen-rebound pathway, wherein a high-valent ruthenium-oxo species abstracts a hydrogen atom from ethylbenzene to generate a radical pair intermediate, followed by the oxygen-rebound process to form 1-phenylethanol. The hydrogen-atom abstraction step is the rate- and stereoselectivity-determining step. Based on the mechanistic model, the computed stereoselectivity is in agreement with the experimental observations. Analysis of the distortion/interaction model suggests that stereoselectivity is determined by both the distortion energy of the ethylbenzene and the interaction energy between the ethylbenzene and the chiral Ru-porphyrin. The steric repulsion between the phenyl group of ethylbenzene and the bulky substituent of Ru-porphyrin is the leading cause of chiral induction.
中文翻译:

Ru-卟啉对苄基CH羟化反应的机理和立体选择性:一项计算研究。
通过密度泛函理论计算,阐明了Ru-卟啉对不对称苄基CH羟基的立体选择性的机理和成因。该反应通过氢原子提取/氧反弹途径进行,其中高价钌-氧物种从乙苯中提取氢原子以生成自由基对中间体,随后进行氧反弹过程以形成1-苯基乙醇。氢原子提取步骤是确定速率和立体选择性的步骤。基于机理模型,计算的立体选择性与实验观察结果一致。对畸变/相互作用模型的分析表明,立体选择性是由乙苯的畸变能和乙苯与手性Ru-卟啉之间的相互作用能共同决定的。乙苯的苯基与Ru-卟啉的大取代基之间的空间排斥是手性诱导的主要原因。
更新日期:2020-01-02
中文翻译:

Ru-卟啉对苄基CH羟化反应的机理和立体选择性:一项计算研究。
通过密度泛函理论计算,阐明了Ru-卟啉对不对称苄基CH羟基的立体选择性的机理和成因。该反应通过氢原子提取/氧反弹途径进行,其中高价钌-氧物种从乙苯中提取氢原子以生成自由基对中间体,随后进行氧反弹过程以形成1-苯基乙醇。氢原子提取步骤是确定速率和立体选择性的步骤。基于机理模型,计算的立体选择性与实验观察结果一致。对畸变/相互作用模型的分析表明,立体选择性是由乙苯的畸变能和乙苯与手性Ru-卟啉之间的相互作用能共同决定的。乙苯的苯基与Ru-卟啉的大取代基之间的空间排斥是手性诱导的主要原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号