当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Uncommon structural and bonding properties in Ag16B4O10
Chemical Science ( IF 7.6 ) Pub Date : 2019/12/09 , DOI: 10.1039/c9sc05185f Anton Kovalevskiy 1 , Congling Yin 1, 2 , Jürgen Nuss 1 , Ulrich Wedig 1 , Martin Jansen 1
Chemical Science ( IF 7.6 ) Pub Date : 2019/12/09 , DOI: 10.1039/c9sc05185f Anton Kovalevskiy 1 , Congling Yin 1, 2 , Jürgen Nuss 1 , Ulrich Wedig 1 , Martin Jansen 1
Affiliation
|
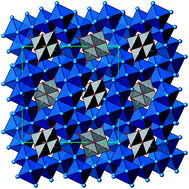
Ag16B4O10 has been obtained as a coarse crystalline material via hydrothermal synthesis, and was characterized by X-ray single crystal and powder diffraction, conductivity and magnetic susceptibility measurements, as well as by DFT based theoretical analyses. Neither composition nor crystal structure nor valence electron counts can be fully rationalized by applying known bonding schemes. While the rare cage anion (B4O10)8− is electron precise, and reflects standard bonding properties, the silver ion substructure necessarily has to accommodate eight excess electrons per formula unit, (Ag+)16(B3+)4(O2−)10 × 8e−, rendering the compound sub-valent with respect to silver. However, the phenomena commonly associated with sub-valence metal (partial) structures are not perceptible in this case. Experimentally, the compound has been found to be semiconducting and diamagnetic, ruling out the presence of itinerant electrons; hence the excess electrons have to localize pairwise. However, no pairwise contractions of silver atoms are realized in the structure, thus excluding formation of 2e–2c bonds. Rather, cluster-like aggregates of an approximately tetrahedral shape exist where the Ag–Ag separations are significantly smaller than in elemental silver. The number of these subunits per formula is four, thus matching the required number of sites for pairwise nesting of eight excess electrons. This scenario has been corroborated by computational analyses of the densities of states and electron localization function (ELF), which clearly indicate the presence of an attractor within the shrunken tetrahedral voids in the silver substructure. However, one bonding electron pair of s and p type skeleton electrons per cluster unit is extremely low, and the significant propensity to form and the thermal stability of the title compound suggest d10–d10 bonding interactions to strengthen the inter-cluster bonding in a synergistic fashion. With the present state of knowledge, such a particular bonding pattern appears to be a singular feature of the oxide chemistry of silver; however, as indicated by analogous findings in related silver oxides, it is evolving as a general one.
中文翻译:

Ag16B4O10 中不常见的结构和键合特性
Ag 16 B 4 O 10已通过水热合成作为粗晶材料获得,并通过 X 射线单晶和粉末衍射、电导率和磁化率测量以及基于 DFT 的理论分析进行表征。通过应用已知的键合方案,无论是组成、晶体结构还是价电子计数都不能完全合理化。虽然稀有笼式阴离子 (B 4 O 10 ) 8-是电子精确的,并反映了标准的键合特性,但银离子亚结构必须在每个分子式单元中容纳八个多余的电子,(Ag + ) 16 (B3+ ) 4 (O 2− ) 10 × 8e −,使化合物相对于银处于亚价状态。然而,在这种情况下,通常与亚价金属(部分)结构相关的现象是不可察觉的。实验表明,该化合物具有半导体性和抗磁性,排除了流动电子的存在。因此多余的电子必须成对地定位。然而,结构中没有实现银原子的成对收缩,因此排除了 2e-2c 键的形成。相反,存在近似四面体形状的簇状聚集体,其中 Ag-Ag 分离明显小于元素银。每个公式的这些亚基的数量是四个,因此匹配所需的八个多余电子成对嵌套的位点数量。这种情况已通过状态密度和电子定位函数 (ELF) 的计算分析得到证实,这清楚地表明在银子结构中收缩的四面体空隙中存在吸引子。然而,每个簇单元的 s 和 p 型骨架电子的一个键合电子对非常低,标题化合物的显着形成倾向和热稳定性表明 d10 -d 10键合相互作用以协同方式加强簇间键合。根据目前的知识水平,这种特殊的键合模式似乎是银氧化物化学的一个独特特征。然而,正如在相关氧化银中的类似发现所表明的那样,它正在发展成为一种普遍现象。
更新日期:2020-02-10
中文翻译:

Ag16B4O10 中不常见的结构和键合特性
Ag 16 B 4 O 10已通过水热合成作为粗晶材料获得,并通过 X 射线单晶和粉末衍射、电导率和磁化率测量以及基于 DFT 的理论分析进行表征。通过应用已知的键合方案,无论是组成、晶体结构还是价电子计数都不能完全合理化。虽然稀有笼式阴离子 (B 4 O 10 ) 8-是电子精确的,并反映了标准的键合特性,但银离子亚结构必须在每个分子式单元中容纳八个多余的电子,(Ag + ) 16 (B3+ ) 4 (O 2− ) 10 × 8e −,使化合物相对于银处于亚价状态。然而,在这种情况下,通常与亚价金属(部分)结构相关的现象是不可察觉的。实验表明,该化合物具有半导体性和抗磁性,排除了流动电子的存在。因此多余的电子必须成对地定位。然而,结构中没有实现银原子的成对收缩,因此排除了 2e-2c 键的形成。相反,存在近似四面体形状的簇状聚集体,其中 Ag-Ag 分离明显小于元素银。每个公式的这些亚基的数量是四个,因此匹配所需的八个多余电子成对嵌套的位点数量。这种情况已通过状态密度和电子定位函数 (ELF) 的计算分析得到证实,这清楚地表明在银子结构中收缩的四面体空隙中存在吸引子。然而,每个簇单元的 s 和 p 型骨架电子的一个键合电子对非常低,标题化合物的显着形成倾向和热稳定性表明 d10 -d 10键合相互作用以协同方式加强簇间键合。根据目前的知识水平,这种特殊的键合模式似乎是银氧化物化学的一个独特特征。然而,正如在相关氧化银中的类似发现所表明的那样,它正在发展成为一种普遍现象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号