Redox Biology ( IF 11.4 ) Pub Date : 2019-12-06 , DOI: 10.1016/j.redox.2019.101400 Nan Shu 1 , Per Hägglund 1 , Huan Cai 1 , Clare L Hawkins 1 , Michael J Davies 1
|
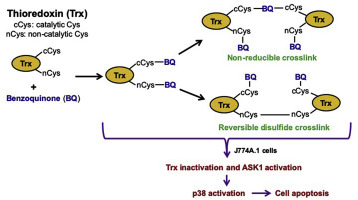
Quinones can modify biological molecules through both redox-cycling reactions that yield radicals (semiquinone, superoxide and hydroxyl) and via covalent adduction to nucleophiles (e.g. thiols and amines). Kinetic data indicate that Cys residues in GSH and proteins are major targets. In the studies reported here, the interactions of a prototypic quinone compound, p-benzoquinone (BQ), with the key redox protein, thioredoxin-1 (Trx1) were examined. BQ binds covalently with isolated Trx1 forming quinoprotein adducts, resulting in a concentration-dependent loss of enzyme activity and crosslink formation. Mass spectrometry peptide mass mapping data indicate that BQ forms adducts with all of the Trx1 Cys residues. Glutathione (GSH) reacts competitively with BQ, and thereby modulates the loss of activity and crosslink formation. Exposure of macrophage-like (J774A.1) cells to BQ results in a dose-dependent loss of Trx and thioredoxin reductase (TrxR) activities, quinoprotein formation, and a decrease in GSH levels without a concomitant increase in oxidized glutathione. GSH depletion aggravates the loss of Trx and TrxR activity. These data are consistent with adduction of GSH to BQ being a primary protective pathway. Reaction of BQ with Trx in cells resulted in the activation of apoptosis signal-regulating kinase 1 (ASK1), and p38 mitogen-activated protein kinase (MAPK) leading to apoptotic cell death. These data suggest that BQ reacts covalently with Cys residues in Trx, including at the active site, leading to enzyme inactivation and protein cross-linking. Modification of the Cys residues in Trx also results in activation of the ASK1/p38-MAPK signalling pathway and promotion of apoptotic cell death.
中文翻译:

对苯醌对人硫氧还蛋白-1中Cys残基的修饰引起其催化活性的抑制和ASK1 / p38-MAPK信号通路的激活。
醌可以通过产生自由基(半醌,超氧化物和羟基)的氧化还原循环反应以及通过亲核试剂(例如硫醇和胺)的共价加成来修饰生物分子。动力学数据表明,GSH和蛋白质中的Cys残基是主要靶标。在这里报道的研究中,原型醌化合物p的相互作用-苯醌(BQ),与关键的氧化还原蛋白,硫氧还蛋白1(Trx1)进行了检查。BQ与分离的Trx1共价结合形成喹啉蛋白加合物,导致酶活性和交联形成的浓度依赖性损失。质谱肽质谱图数据表明BQ与所有Trx1 Cys残基形成加合物。谷胱甘肽(GSH)与BQ竞争性反应,从而调节活性的丧失和交联的形成。巨噬细胞样(J774A.1)细胞暴露于BQ会导致Trx和硫氧还蛋白还原酶(TrxR)活性的剂量依赖性损失,喹诺酮蛋白的形成和GSH含量的降低,而氧化型谷胱甘肽却没有随之增加。GSH耗竭加剧了Trx和TrxR活性的丧失。这些数据与将GSH加到BQ是主要的保护途径是一致的。BQ与Trx在细胞中的反应导致凋亡信号调节激酶1(ASK1)和p38丝裂原活化蛋白激酶(MAPK)的激活,导致凋亡性细胞死亡。这些数据表明BQ与Trx中的Cys残基共价反应,包括在活性位点,导致酶失活和蛋白质交联。Trx中Cys残基的修饰还导致ASK1 / p38-MAPK信号通路的激活和凋亡细胞死亡的促进。



























 京公网安备 11010802027423号
京公网安备 11010802027423号