当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of membrane composition on DivIVA-membrane interaction.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2019-12-07 , DOI: 10.1016/j.bbamem.2019.183144 Miroslav Jurásek 1 , Klas Flärdh 2 , Robert Vácha 3
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2019-12-07 , DOI: 10.1016/j.bbamem.2019.183144 Miroslav Jurásek 1 , Klas Flärdh 2 , Robert Vácha 3
Affiliation
|
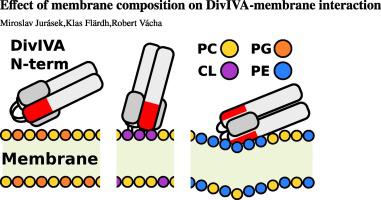
DivIVA is a crucial membrane-binding protein that helps to localize other proteins to negatively curved membranes at cellular poles and division septa in Gram-positive bacteria. The N-terminal domain of DivIVA is responsible for membrane binding. However, to which lipids the domain binds or how it recognizes the membrane negative curvature remains elusive. Using computer simulations, we demonstrate that the N-terminal domain of Streptomyces coelicolor DivIVA adsorbs to membranes with affinity and orientation dependent on the lipid composition. The domain interacts non-specifically with lipid phosphates via its arginine-rich tip and the strongest interaction is with cardiolipin. Moreover, we observed a specific attraction between a negatively charged side patch of the domain and ethanolamine lipids, which addition caused the change of the domain orientation from perpendicular to parallel alignment to the membrane plane. Similar but less electrostatically dependent behavior was observed for the N-terminal domain of Bacillus subtilis. The domain propensity for lipids which prefer negatively curved membranes could be a mechanism for the cellular localization of DivIVA protein.
中文翻译:

膜组成对DivIVA-膜相互作用的影响。
DivIVA是一种至关重要的膜结合蛋白,可帮助将其他蛋白定位在革兰氏阳性细菌的细胞极和分裂间隔的负弯曲膜上。DivIVA的N末端域负责膜结合。但是,该域结合在哪些脂质上或如何识别膜的负曲率仍然难以捉摸。使用计算机模拟,我们证明链霉菌天蓝色链霉菌DivIVA的N末端域吸附到膜的亲和力和方向取决于脂质的组成。该结构域通过其富含精氨酸的尖端与脂质磷酸酯非特异性相互作用,而最强的相互作用是与心磷脂。此外,我们观察到该域的带负电荷的侧片与乙醇胺脂质之间有特定的吸引力,该添加导致畴取向从垂直到平行于膜平面取向改变。对于枯草芽孢杆菌的N-末端结构域,观察到相似但较少的静电依赖性行为。偏向负弯曲膜的脂质的结构域倾向可能是DivIVA蛋白在细胞内定位的一种机制。
更新日期:2019-12-07
中文翻译:

膜组成对DivIVA-膜相互作用的影响。
DivIVA是一种至关重要的膜结合蛋白,可帮助将其他蛋白定位在革兰氏阳性细菌的细胞极和分裂间隔的负弯曲膜上。DivIVA的N末端域负责膜结合。但是,该域结合在哪些脂质上或如何识别膜的负曲率仍然难以捉摸。使用计算机模拟,我们证明链霉菌天蓝色链霉菌DivIVA的N末端域吸附到膜的亲和力和方向取决于脂质的组成。该结构域通过其富含精氨酸的尖端与脂质磷酸酯非特异性相互作用,而最强的相互作用是与心磷脂。此外,我们观察到该域的带负电荷的侧片与乙醇胺脂质之间有特定的吸引力,该添加导致畴取向从垂直到平行于膜平面取向改变。对于枯草芽孢杆菌的N-末端结构域,观察到相似但较少的静电依赖性行为。偏向负弯曲膜的脂质的结构域倾向可能是DivIVA蛋白在细胞内定位的一种机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号