当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural insights into the active site of poly(ADP-ribose) glycohydrolase using docking modes of 6-hydroxy-3H-xanthen-3-one derivative inhibitors.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-06 , DOI: 10.1016/j.bmc.2019.115249 Yuto Shibui 1 , Takahiro Oyama 2 , Miwa Okazawa 3 , Atsushi Yoshimori 4 , Hideaki Abe 2 , Fumiaki Uchiumi 1 , Sei-Ichi Tanuma 3
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-06 , DOI: 10.1016/j.bmc.2019.115249 Yuto Shibui 1 , Takahiro Oyama 2 , Miwa Okazawa 3 , Atsushi Yoshimori 4 , Hideaki Abe 2 , Fumiaki Uchiumi 1 , Sei-Ichi Tanuma 3
Affiliation
|
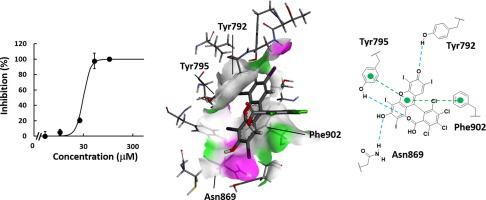
Poly(ADP-ribose) glycohydrolase (PARG) plays an essential role in poly(ADP-ribose) (PAR) turnover, and thereby regulating DNA transactions, such as DNA repair, replication, transcription and recombination. Here, we examined the inhibitory activities of 6-hydroxy-3H-xanthene-3-one (HXO) derivatives and analyzed their binding modes in the active site of PARG by in silico docking study. Among the derivatives, Rose Bengal was found to be the most potent inhibitor of PARG and its halogen groups were revealed to cooperatively potentiate the inhibitory activity. Importantly, the binding mode of Rose Bengal occupied the active site of PARG revealed the presence of unique "Sandwich" residues of Asn869 and Tyr792, which enable the inhibitor to bind tightly with the active pocket. This sandwich interaction could stabilize the π-π interactions of HXO scaffold with Phe902 and Tyr795. In addition, to increase the binding affinity, the iodine and chlorine atoms of this inhibitor could contribute to the inducing of favorable disorders, which promote an entropy boost on the active site of PARG for structural plasticity, and making the stable configuration of HXO scaffold in the active site, respectively, as judged by the analysis of binding free energy. These results provide new insights into the active site of PARG and an additional opportunity for designing selective PARG inhibitors.
中文翻译:

使用6-羟基-3H-黄嘌呤-3-酮衍生物抑制剂的对接模式,深入了解聚(ADP-核糖)糖水解酶的活性位点。
聚(ADP-核糖)糖水解酶(PARG)在聚(ADP-核糖)(PAR)周转中起至关重要的作用,从而调节DNA交易,例如DNA修复,复制,转录和重组。在这里,我们通过计算机对接研究检查了6-羟基-3H--吨-3-酮(HXO)衍生物的抑制活性,并分析了它们在PARG活性位点的结合方式。在衍生物中,Rose Bengal被发现是最有效的PARG抑制剂,并且其卤素基团显示出协同增强了抑制活性。重要的是,Rose Bengal的结合模式占据了PARG的活性位点,揭示了Asn869和Tyr792独特的“三明治”残基的存在,这些残基使抑制剂能够与活性囊紧密结合。这种三明治相互作用可以稳定HXO支架与Phe902和Tyr795的π-π相互作用。此外,为增加结合亲和力,该抑制剂的碘和氯原子可能有助于诱导有利的疾病,从而促进PARG活性位点的熵增强,从而增强结构可塑性,并使HXO支架的结构稳定。通过结合自由能的分析判断活性位点。这些结果为了解PARG的活性位点提供了新的见识,并为设计选择性PARG抑制剂提供了额外的机会。通过结合自由能的分析判断,它们分别促进了PARG活性位点的熵增强以增强结构可塑性,并使HXO支架在活性位点处具有稳定的构型。这些结果为了解PARG的活性位点提供了新的见识,为设计选择性PARG抑制剂提供了额外的机会。通过结合自由能的分析判断,它们分别促进了PARG活性位点的熵增强以增强结构可塑性,并使HXO支架在活性位点处具有稳定的构型。这些结果为了解PARG的活性位点提供了新的见识,为设计选择性PARG抑制剂提供了额外的机会。
更新日期:2019-12-07
中文翻译:

使用6-羟基-3H-黄嘌呤-3-酮衍生物抑制剂的对接模式,深入了解聚(ADP-核糖)糖水解酶的活性位点。
聚(ADP-核糖)糖水解酶(PARG)在聚(ADP-核糖)(PAR)周转中起至关重要的作用,从而调节DNA交易,例如DNA修复,复制,转录和重组。在这里,我们通过计算机对接研究检查了6-羟基-3H--吨-3-酮(HXO)衍生物的抑制活性,并分析了它们在PARG活性位点的结合方式。在衍生物中,Rose Bengal被发现是最有效的PARG抑制剂,并且其卤素基团显示出协同增强了抑制活性。重要的是,Rose Bengal的结合模式占据了PARG的活性位点,揭示了Asn869和Tyr792独特的“三明治”残基的存在,这些残基使抑制剂能够与活性囊紧密结合。这种三明治相互作用可以稳定HXO支架与Phe902和Tyr795的π-π相互作用。此外,为增加结合亲和力,该抑制剂的碘和氯原子可能有助于诱导有利的疾病,从而促进PARG活性位点的熵增强,从而增强结构可塑性,并使HXO支架的结构稳定。通过结合自由能的分析判断活性位点。这些结果为了解PARG的活性位点提供了新的见识,并为设计选择性PARG抑制剂提供了额外的机会。通过结合自由能的分析判断,它们分别促进了PARG活性位点的熵增强以增强结构可塑性,并使HXO支架在活性位点处具有稳定的构型。这些结果为了解PARG的活性位点提供了新的见识,为设计选择性PARG抑制剂提供了额外的机会。通过结合自由能的分析判断,它们分别促进了PARG活性位点的熵增强以增强结构可塑性,并使HXO支架在活性位点处具有稳定的构型。这些结果为了解PARG的活性位点提供了新的见识,为设计选择性PARG抑制剂提供了额外的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号