Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antenatal magnesium sulphate and adverse neonatal outcomes: A systematic review and meta-analysis.
PLOS Medicine ( IF 10.5 ) Pub Date : 2019-12-06 , DOI: 10.1371/journal.pmed.1002988 Emily Shepherd 1, 2 , Rehana A Salam 1, 2 , Deepak Manhas 3 , Anne Synnes 3 , Philippa Middleton 1, 2 , Maria Makrides 2 , Caroline A Crowther 1, 4
PLOS Medicine ( IF 10.5 ) Pub Date : 2019-12-06 , DOI: 10.1371/journal.pmed.1002988 Emily Shepherd 1, 2 , Rehana A Salam 1, 2 , Deepak Manhas 3 , Anne Synnes 3 , Philippa Middleton 1, 2 , Maria Makrides 2 , Caroline A Crowther 1, 4
Affiliation
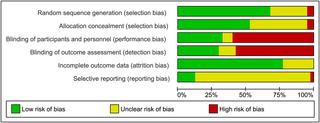
|
BACKGROUND
There is widespread, increasing use of magnesium sulphate in obstetric practice for pre-eclampsia, eclampsia, and preterm fetal neuroprotection; benefit for preventing preterm labour and birth (tocolysis) is unproven. We conducted a systematic review and meta-analysis to assess whether antenatal magnesium sulphate is associated with unintended adverse neonatal outcomes.
METHODS AND FINDINGS
CINAHL, Cochrane Library, LILACS, MEDLINE, Embase, TOXLINE, and Web of Science, were searched (inceptions to 3 September 2019). Randomised, quasi-randomised, and non-randomised trials, cohort and case-control studies, and case reports assessing antenatal magnesium sulphate for pre-eclampsia, eclampsia, fetal neuroprotection, or tocolysis, compared with placebo/no treatment or a different magnesium sulphate regimen, were included. The primary outcome was perinatal death. Secondary outcomes included pre-specified and non-pre-specified adverse neonatal outcomes. Two reviewers screened 5,890 articles, extracted data, and assessed risk of bias following Cochrane Handbook and RTI Item Bank guidance. For randomised trials, pooled risk ratios (RRs) or mean differences, with 95% confidence intervals (CIs), were calculated using fixed- or random-effects meta-analysis. Non-randomised data were tabulated and narratively summarised. We included 197 studies (40 randomised trials, 138 non-randomised studies, and 19 case reports), of mixed quality. The 40 trials (randomising 19,265 women and their babies) were conducted from 1987 to 2018 across high- (16 trials) and low/middle-income countries (23 trials) (1 mixed). Indications included pre-eclampsia/eclampsia (24 trials), fetal neuroprotection (7 trials), and tocolysis (9 trials); 18 trials compared magnesium sulphate with placebo/no treatment, and 22 compared different regimens. For perinatal death, no clear difference in randomised trials was observed between magnesium sulphate and placebo/no treatment (RR 1.01; 95% CI 0.92 to 1.10; 8 trials, 13,654 babies), nor between regimens. Eleven of 138 non-randomised studies reported on perinatal death. Only 1 cohort (127 babies; moderate to high risk of bias) observed an increased risk of perinatal death with >48 versus ≤48 grams magnesium sulphate exposure for tocolysis. No clear secondary adverse neonatal outcomes were observed in randomised trials, and a very limited number of possible adverse outcomes warranting further consideration were identified in non-randomised studies. Where non-randomised studies observed possible harms, often no or few confounders were controlled for (moderate to high risk of bias), samples were small (200 babies or fewer), and/or results were from subgroup analyses. Limitations include missing data for important outcomes across most studies, heterogeneity of included studies, and inclusion of published data only.
CONCLUSIONS
Our findings do not support clear associations between antenatal magnesium sulphate for beneficial indications and adverse neonatal outcomes. Further large, high-quality studies (prospective cohorts or individual participant data meta-analyses) assessing specific outcomes, or the impact of regimen, pregnancy, or birth characteristics on these outcomes, would further inform safety recommendations. PROSPERO: CRD42013004451.
中文翻译:

产前硫酸镁和不良新生儿结局:系统评价和荟萃分析。
背景技术硫酸镁在产科实践中广泛且越来越多地用于治疗先兆子痫、子痫和早产儿神经保护;预防早产和分娩(安胎)的益处尚未得到证实。我们进行了系统评价和荟萃分析,以评估产前硫酸镁是否与意外的不良新生儿结局相关。方法和结果 检索了 CINAHL、Cochrane Library、LILACS、MEDLINE、Embase、TOXLINE 和 Web of Science(起始日期截至 2019 年 9 月 3 日)。与安慰剂/不治疗或不同的硫酸镁相比,评估产前硫酸镁对先兆子痫、子痫、胎儿神经保护或安胎的随机、半随机和非随机试验、队列和病例对照研究以及病例报告方案,包括在内。主要结局是围产期死亡。次要结局包括预先指定的和非预先指定的不良新生儿结局。两名审稿人筛选了 5,890 篇文章,提取数据,并按照 Cochrane 手册和 RTI 项目库指导评估偏倚风险。对于随机试验,使用固定效应或随机效应荟萃分析计算汇总风险比 (RR) 或平均差异(95% 置信区间 (CI))。将非随机数据制成表格并进行叙述性总结。我们纳入了 197 项研究(40 项随机试验、138 项非随机研究和 19 项病例报告),质量参差不齐。这 40 项试验(随机分配 19,265 名妇女及其婴儿)于 1987 年至 2018 年在高收入国家(16 项试验)和低/中等收入国家(23 项试验)(1 项混合试验)进行。适应症包括先兆子痫/子痫(24 项试验)、胎儿神经保护(7 项试验)和安胎(9 项试验);18 项试验将硫酸镁与安慰剂/无治疗进行比较,22 项试验比较不同的治疗方案。对于围产期死亡,随机试验中硫酸镁和安慰剂/不治疗之间没有观察到明显差异(RR 1.01;95% CI 0.92 至 1.10;8 项试验,13,654 名婴儿),治疗方案之间也没有观察到明显差异。138 项非随机研究中有 11 项报告了围产期死亡。只有 1 个队列(127 名婴儿;中度至高偏倚风险)观察到安胎时暴露 >48 克与≤48 克硫酸镁会增加围产期死亡风险。随机试验中没有观察到明确的继发性新生儿不良结局,非随机研究中发现了非常有限的需要进一步考虑的可能不良结局。非随机研究观察到可能的危害,通常没有或很少控制混杂因素(中度至高偏倚风险),样本较小(200 名婴儿或更少),和/或结果来自亚组分析。局限性包括大多数研究中重要结果的数据缺失、纳入研究的异质性以及仅包含已发表的数据。结论 我们的研究结果并不支持产前硫酸镁的有益适应症与不良新生儿结局之间存在明确关联。评估特定结果或治疗方案、妊娠或出生特征对这些结果的影响的进一步大型高质量研究(前瞻性队列或个体参与者数据荟萃分析)将进一步为安全建议提供信息。普洛斯彼罗:CRD42013004451。
更新日期:2020-01-14
中文翻译:

产前硫酸镁和不良新生儿结局:系统评价和荟萃分析。
背景技术硫酸镁在产科实践中广泛且越来越多地用于治疗先兆子痫、子痫和早产儿神经保护;预防早产和分娩(安胎)的益处尚未得到证实。我们进行了系统评价和荟萃分析,以评估产前硫酸镁是否与意外的不良新生儿结局相关。方法和结果 检索了 CINAHL、Cochrane Library、LILACS、MEDLINE、Embase、TOXLINE 和 Web of Science(起始日期截至 2019 年 9 月 3 日)。与安慰剂/不治疗或不同的硫酸镁相比,评估产前硫酸镁对先兆子痫、子痫、胎儿神经保护或安胎的随机、半随机和非随机试验、队列和病例对照研究以及病例报告方案,包括在内。主要结局是围产期死亡。次要结局包括预先指定的和非预先指定的不良新生儿结局。两名审稿人筛选了 5,890 篇文章,提取数据,并按照 Cochrane 手册和 RTI 项目库指导评估偏倚风险。对于随机试验,使用固定效应或随机效应荟萃分析计算汇总风险比 (RR) 或平均差异(95% 置信区间 (CI))。将非随机数据制成表格并进行叙述性总结。我们纳入了 197 项研究(40 项随机试验、138 项非随机研究和 19 项病例报告),质量参差不齐。这 40 项试验(随机分配 19,265 名妇女及其婴儿)于 1987 年至 2018 年在高收入国家(16 项试验)和低/中等收入国家(23 项试验)(1 项混合试验)进行。适应症包括先兆子痫/子痫(24 项试验)、胎儿神经保护(7 项试验)和安胎(9 项试验);18 项试验将硫酸镁与安慰剂/无治疗进行比较,22 项试验比较不同的治疗方案。对于围产期死亡,随机试验中硫酸镁和安慰剂/不治疗之间没有观察到明显差异(RR 1.01;95% CI 0.92 至 1.10;8 项试验,13,654 名婴儿),治疗方案之间也没有观察到明显差异。138 项非随机研究中有 11 项报告了围产期死亡。只有 1 个队列(127 名婴儿;中度至高偏倚风险)观察到安胎时暴露 >48 克与≤48 克硫酸镁会增加围产期死亡风险。随机试验中没有观察到明确的继发性新生儿不良结局,非随机研究中发现了非常有限的需要进一步考虑的可能不良结局。非随机研究观察到可能的危害,通常没有或很少控制混杂因素(中度至高偏倚风险),样本较小(200 名婴儿或更少),和/或结果来自亚组分析。局限性包括大多数研究中重要结果的数据缺失、纳入研究的异质性以及仅包含已发表的数据。结论 我们的研究结果并不支持产前硫酸镁的有益适应症与不良新生儿结局之间存在明确关联。评估特定结果或治疗方案、妊娠或出生特征对这些结果的影响的进一步大型高质量研究(前瞻性队列或个体参与者数据荟萃分析)将进一步为安全建议提供信息。普洛斯彼罗:CRD42013004451。











































 京公网安备 11010802027423号
京公网安备 11010802027423号