当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Apomorphine sublingual film for off episodes in Parkinson's disease: a randomised, double-blind, placebo-controlled phase 3 study
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-02-01 , DOI: 10.1016/s1474-4422(19)30396-5 C Warren Olanow 1 , Stewart A Factor 2 , Alberto J Espay 3 , Robert A Hauser 4 , Holly A Shill 5 , Stuart Isaacson 6 , Rajesh Pahwa 7 , Mika Leinonen 8 , Parul Bhargava 9 , Ken Sciarappa 9 , Bradford Navia 9 , David Blum 9 ,
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-02-01 , DOI: 10.1016/s1474-4422(19)30396-5 C Warren Olanow 1 , Stewart A Factor 2 , Alberto J Espay 3 , Robert A Hauser 4 , Holly A Shill 5 , Stuart Isaacson 6 , Rajesh Pahwa 7 , Mika Leinonen 8 , Parul Bhargava 9 , Ken Sciarappa 9 , Bradford Navia 9 , David Blum 9 ,
Affiliation
|
BACKGROUND
Many patients with Parkinson's disease have potentially disabling off episodes that are not predictably responsive to levodopa. In this study, we assessed the safety and efficacy of apomorphine sublingual film as an on-demand therapy for off episodes in patients with Parkinson's disease. METHODS
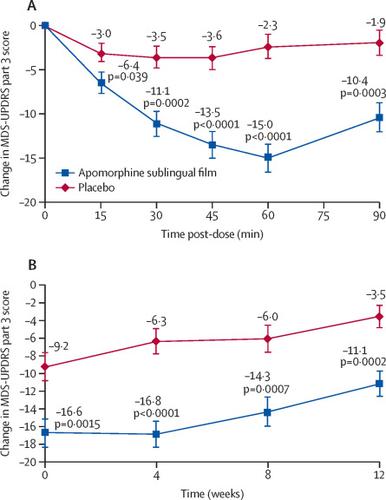
This randomised, double-blind, placebo-controlled study was done by movement disorder specialists at 32 sites in the USA and one in Canada. Patients with Parkinson's disease who had 2 h or more of off time per day with predictable morning off periods, were responsive to levodopa, and were on stable doses of anti-parkinsonian medication were eligible. In an open-label titration phase, increasing doses of apomorphine sublingual film (10-35 mg) were administered until a tolerable full on response was achieved. Patients were then randomly assigned (1:1) with an interactive web-response system to receive the effective dose of apomorphine sublingual film or matching placebo in a 12-week, double-blind maintenance phase. Randomisation was not stratified, and the block size was four. All patients and study personnel were masked to treatment assignments. The primary endpoint was the in-clinic change from predose to 30 min post-dose in the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) part 3 (motor) score at week 12, analysed on a modified intention-to-treat population by use of a mixed-effect model for repeated measures. Safety analyses were done on all enrolled patients who received at least one dose of study medication. This trial is registered with ClinicalTrials.gov, NCT02469090. FINDINGS
Between June 18, 2015, and Dec 11, 2017, 109 patients were enrolled and randomly assigned to receive apomorphine sublingual film (n=54) or placebo (n=55). All patients received the assigned study treatment, and 34 (63%) of 54 patients receiving apomorphine sublingual film and 46 (84%) of 55 receiving placebo completed the study. Least squares mean (SE) change from predose to 30 min post-dose in MDS-UPDRS part 3 score at week 12 was -11·1 (SE 1·46, 95% CI -14·0 to -8·2) with apomorphine sublingual film and -3·5 (1·29, -6·1 to -0·9) with placebo (difference -7·6, SE 1·96, 95% CI -11·5 to -3·7; p=0·0002). Mild-to-moderate oropharyngeal events were the most common side-effect, reported in 17 (31%) of 54 patients receiving apomorphine sublingual film and in four (7%) of 55 patients receiving placebo, leading to treatment discontinuation in nine (17%) patients treated with apomorphine and in one (2%) patient treated with placebo. Other treatment-emergent adverse events were transient nausea (in 15 [28%] patients receiving apomorphine sublingual film), somnolence (seven [13%]), and dizziness (five [9%]). Orthostatic hypotension, syncope, dyskinesia, hallucinations, prolongation of the QT interval, and impulse control disorders were infrequent (prevalence ≤2% of all patients) or did not occur. One patient treated with apomorphine sublingual film (with known cardiac risk factors) had a fatal cardiac arrest. INTERPRETATION
Although nearly a third of patients discontinued treatment primarily because of oropharyngeal side-effects, apomorphine sublingual film provided an efficacious, on-demand treatment for off episodes for most patients with Parkinson's disease in this trial. The long-term safety and efficacy of apomorphine sublingual film are currently being investigated. FUNDING
Cynapsus Therapeutics and Sunovion.
中文翻译:

阿扑吗啡舌下膜用于帕金森病发作:一项随机、双盲、安慰剂对照的 3 期研究
背景 许多患有帕金森氏病的患者可能会禁用对左旋多巴无反应的发作。在这项研究中,我们评估了阿扑吗啡舌下膜作为帕金森病患者间歇性发作的按需疗法的安全性和有效性。方法 这项随机、双盲、安慰剂对照研究由运动障碍专家在美国的 32 个地点和加拿大的一个地点进行。每天有 2 小时或更长时间的休息时间和可预测的早晨休息时间、对左旋多巴有反应并且服用稳定剂量的抗帕金森病药物的帕金森病患者符合条件。在开放标签滴定阶段,增加剂量的阿扑吗啡舌下膜(10-35 毫克)直至达到可耐受的完全反应。然后将患者随机分配 (1:1) 使用交互式网络响应系统,在 12 周的双盲维持阶段接受有效剂量的阿扑吗啡舌下膜或匹配的安慰剂。随机化未分层,块大小为 4。所有患者和研究人员都对治疗分配不知情。主要终点是第 12 周运动障碍协会统一帕金森病评定量表 (MDS-UPDRS) 第 3 部分(运动)评分中从给药前到给药后 30 分钟的临床变化,根据修改后的意向性进行分析 -通过使用重复测量的混合效应模型来处理人口。对所有接受至少一剂研究药物的入选患者进行安全性分析。该试验已在 ClinicalTrials.gov 注册,NCT02469090。调查结果 2015 年 6 月 18 日,2017 年 12 月 11 日,109 名患者被纳入并随机分配接受阿扑吗啡舌下膜(n=54)或安慰剂(n=55)。所有患者都接受了指定的研究治疗,接受阿扑吗啡舌下膜的 54 名患者中有 34 名 (63%) 和接受安慰剂的 55 名患者中有 46 名 (84%) 完成了研究。在第 12 周的 MDS-UPDRS 第 3 部分评分中,从给药前到给药后 30 分钟的最小二乘均值 (SE) 变化为 -11·1(SE 1·46,95% CI -14·0 到 -8·2),其中阿扑吗啡舌下膜片和 -3·5(1·29,-6·1 至 -0·9)与安慰剂(差异 -7·6,SE 1·96,95% CI -11·5 至 -3·7; p=0·0002)。轻度至中度口咽事件是最常见的副作用,据报道,接受阿扑吗啡舌下膜的 54 名患者中有 17 名 (31%) 和接受安慰剂的 55 名患者中有 4 名 (7%),导致 9 名 (17%) 接受阿扑吗啡治疗的患者和 1 名 (2%) 接受安慰剂治疗的患者停止治疗。其他治疗中出现的不良事件包括一过性恶心(15 名 [28%] 接受阿扑吗啡舌下膜的患者)、嗜睡(7 名 [13%])和头晕(5 名 [9%])。体位性低血压、晕厥、运动障碍、幻觉、QT 间期延长和冲动控制障碍不常见(患病率≤所有患者的 2%)或没有发生。一名接受阿扑吗啡舌下膜(具有已知心脏危险因素)治疗的患者出现了致命的心脏骤停。解释 尽管近三分之一的患者主要因为口咽副作用而停止治疗,但阿扑吗啡舌下膜提供了一种有效的、在该试验中,大多数帕金森病患者的停药期按需治疗。目前正在研究阿扑吗啡舌下膜的长期安全性和有效性。资助 Cynapsus Therapeutics 和 Sunovion。
更新日期:2020-02-01
中文翻译:

阿扑吗啡舌下膜用于帕金森病发作:一项随机、双盲、安慰剂对照的 3 期研究
背景 许多患有帕金森氏病的患者可能会禁用对左旋多巴无反应的发作。在这项研究中,我们评估了阿扑吗啡舌下膜作为帕金森病患者间歇性发作的按需疗法的安全性和有效性。方法 这项随机、双盲、安慰剂对照研究由运动障碍专家在美国的 32 个地点和加拿大的一个地点进行。每天有 2 小时或更长时间的休息时间和可预测的早晨休息时间、对左旋多巴有反应并且服用稳定剂量的抗帕金森病药物的帕金森病患者符合条件。在开放标签滴定阶段,增加剂量的阿扑吗啡舌下膜(10-35 毫克)直至达到可耐受的完全反应。然后将患者随机分配 (1:1) 使用交互式网络响应系统,在 12 周的双盲维持阶段接受有效剂量的阿扑吗啡舌下膜或匹配的安慰剂。随机化未分层,块大小为 4。所有患者和研究人员都对治疗分配不知情。主要终点是第 12 周运动障碍协会统一帕金森病评定量表 (MDS-UPDRS) 第 3 部分(运动)评分中从给药前到给药后 30 分钟的临床变化,根据修改后的意向性进行分析 -通过使用重复测量的混合效应模型来处理人口。对所有接受至少一剂研究药物的入选患者进行安全性分析。该试验已在 ClinicalTrials.gov 注册,NCT02469090。调查结果 2015 年 6 月 18 日,2017 年 12 月 11 日,109 名患者被纳入并随机分配接受阿扑吗啡舌下膜(n=54)或安慰剂(n=55)。所有患者都接受了指定的研究治疗,接受阿扑吗啡舌下膜的 54 名患者中有 34 名 (63%) 和接受安慰剂的 55 名患者中有 46 名 (84%) 完成了研究。在第 12 周的 MDS-UPDRS 第 3 部分评分中,从给药前到给药后 30 分钟的最小二乘均值 (SE) 变化为 -11·1(SE 1·46,95% CI -14·0 到 -8·2),其中阿扑吗啡舌下膜片和 -3·5(1·29,-6·1 至 -0·9)与安慰剂(差异 -7·6,SE 1·96,95% CI -11·5 至 -3·7; p=0·0002)。轻度至中度口咽事件是最常见的副作用,据报道,接受阿扑吗啡舌下膜的 54 名患者中有 17 名 (31%) 和接受安慰剂的 55 名患者中有 4 名 (7%),导致 9 名 (17%) 接受阿扑吗啡治疗的患者和 1 名 (2%) 接受安慰剂治疗的患者停止治疗。其他治疗中出现的不良事件包括一过性恶心(15 名 [28%] 接受阿扑吗啡舌下膜的患者)、嗜睡(7 名 [13%])和头晕(5 名 [9%])。体位性低血压、晕厥、运动障碍、幻觉、QT 间期延长和冲动控制障碍不常见(患病率≤所有患者的 2%)或没有发生。一名接受阿扑吗啡舌下膜(具有已知心脏危险因素)治疗的患者出现了致命的心脏骤停。解释 尽管近三分之一的患者主要因为口咽副作用而停止治疗,但阿扑吗啡舌下膜提供了一种有效的、在该试验中,大多数帕金森病患者的停药期按需治疗。目前正在研究阿扑吗啡舌下膜的长期安全性和有效性。资助 Cynapsus Therapeutics 和 Sunovion。











































 京公网安备 11010802027423号
京公网安备 11010802027423号