当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of N -pyridoyl-Δ2 -pyrazolines as Hsp90 inhibitors
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2019-12-06 , DOI: 10.1002/ardp.201900192 Sundeep Kadasi 1, 2 , Ravali Yerroju 1 , Swetha Gaddam 1 , Nikhila Pullanagiri 1 , Meghana Chary 1 , Divya Pingili 3 , Shiva Raj 2 , Nulgumnalli Manjunathaiah Raghavendra 1, 4
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2019-12-06 , DOI: 10.1002/ardp.201900192 Sundeep Kadasi 1, 2 , Ravali Yerroju 1 , Swetha Gaddam 1 , Nikhila Pullanagiri 1 , Meghana Chary 1 , Divya Pingili 3 , Shiva Raj 2 , Nulgumnalli Manjunathaiah Raghavendra 1, 4
Affiliation
|
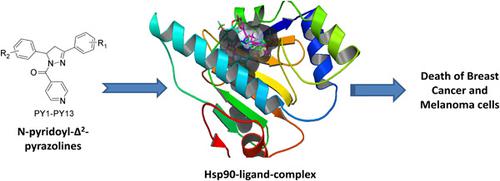
Hsp90, as a key molecular chaperone, plays an important role in modulating the activity of many cell signaling proteins and is an attractive target for anticancer therapeutics. Herein, we report the discovery of N‐pyridoyl‐Δ2‐pyrazoline analogs as novel Hsp90 inhibitors by integrated approaches of drug design, organic synthesis, cell biology, and qualitative proteomic analysis. Novel chemical compounds were designed and optimized in the adenosine triphosphate‐binding site of Hsp90; lead optimized compounds were found to have significant interactions with Asp93 and other amino acids crucial for Hsp90 inhibition. The designed compounds were synthesized by a two‐step procedure; different aromatic aldehydes were reacted with various acetophenones to form substituted 1,3‐diphenyl‐prop‐2‐enones (Ic–Io), which upon reaction with isonicotinic acid hydrazide in the presence of glacial acetic acid form N‐pyridoyl‐Δ2‐pyrazoline compounds (PY1–PY13). Compounds PY3, PY2, and PY1 were identified as potential leads amongst the series, with promising anticancer activity against human breast cancer and melanoma cells, and the ability to inhibit Hsp90 similar to radicicol by drug‐affinity responsive target stability proteomic analysis in a whole‐cell assay.
中文翻译:

发现 N-吡啶酰基-Δ2-吡唑啉作为 Hsp90 抑制剂
Hsp90 作为一种关键的分子伴侣,在调节许多细胞信号蛋白的活性方面发挥着重要作用,并且是抗癌治疗的一个有吸引力的靶点。在此,我们通过药物设计、有机合成、细胞生物学和定性蛋白质组学分析的综合方法,报告了作为新型 Hsp90 抑制剂的 N-吡啶基-Δ2-吡唑啉类似物的发现。在 Hsp90 的三磷酸腺苷结合位点设计和优化了新的化合物;发现先导优化化合物与 Asp93 和其他对 Hsp90 抑制至关重要的氨基酸具有显着的相互作用。设计的化合物通过两步程序合成;不同的芳香醛与各种苯乙酮反应形成取代的 1,3-二苯基-丙-2-烯酮 (Ic-Io),在冰醋酸存在下与异烟酸酰肼反应形成 N-吡啶基-Δ2-吡唑啉化合物(PY1-PY13)。化合物 PY3、PY2 和 PY1 被确定为该系列中的潜在先导物,对人乳腺癌和黑色素瘤细胞具有良好的抗癌活性,并且通过药物亲和反应靶标稳定性蛋白质组学分析,具有抑制 Hsp90 的能力细胞测定。
更新日期:2019-12-06
中文翻译:

发现 N-吡啶酰基-Δ2-吡唑啉作为 Hsp90 抑制剂
Hsp90 作为一种关键的分子伴侣,在调节许多细胞信号蛋白的活性方面发挥着重要作用,并且是抗癌治疗的一个有吸引力的靶点。在此,我们通过药物设计、有机合成、细胞生物学和定性蛋白质组学分析的综合方法,报告了作为新型 Hsp90 抑制剂的 N-吡啶基-Δ2-吡唑啉类似物的发现。在 Hsp90 的三磷酸腺苷结合位点设计和优化了新的化合物;发现先导优化化合物与 Asp93 和其他对 Hsp90 抑制至关重要的氨基酸具有显着的相互作用。设计的化合物通过两步程序合成;不同的芳香醛与各种苯乙酮反应形成取代的 1,3-二苯基-丙-2-烯酮 (Ic-Io),在冰醋酸存在下与异烟酸酰肼反应形成 N-吡啶基-Δ2-吡唑啉化合物(PY1-PY13)。化合物 PY3、PY2 和 PY1 被确定为该系列中的潜在先导物,对人乳腺癌和黑色素瘤细胞具有良好的抗癌活性,并且通过药物亲和反应靶标稳定性蛋白质组学分析,具有抑制 Hsp90 的能力细胞测定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号