当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Biases of α-Synuclein and Formation of Transient Knots.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-12-20 , DOI: 10.1021/acs.jpcb.9b08481 Mateusz Chwastyk 1 , Marek Cieplak 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-12-20 , DOI: 10.1021/acs.jpcb.9b08481 Mateusz Chwastyk 1 , Marek Cieplak 1
Affiliation
|
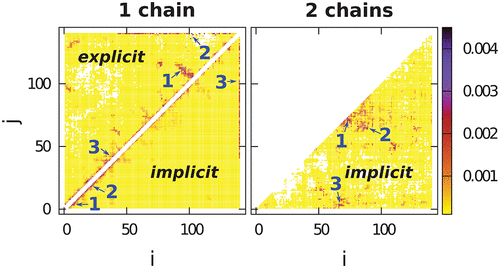
We study local conformational biases in the dynamics of α-synuclein by using all-atom simulations with explicit and implicit solvents. The biases are related to the frequency of the specific contact formation. In both approaches, the protein is intrinsically disordered, and its strongest bias is to make bend and turn local structures. The explicit-solvent conformations can be substantially more extended which allows for formation of transient trefoil knots, both deep and shallow, that may last for up to 5 μs. The two-chain self-association events, both short- and long-lived, are dominated by formation of contacts in the central part of the sequence. This part tends to form helices when bound to a micelle.
中文翻译:

α-突触核蛋白的构象偏差和瞬时结的形成。
我们通过使用具有显性和隐性溶剂的全原子模拟研究α-突触核蛋白动力学的局部构象偏倚。偏压与特定触点形成的频率有关。在这两种方法中,蛋白质都是内在无序的,其最强的偏见是使局部结构弯曲和转向。显式溶剂构象可以大大扩展,从而允许形成深和浅的短暂三叶形结,持续时间长达5μs。短寿命和长寿命的两链自缔合事件主要由序列中心部分的接触形成所决定。当与胶束结合时,该部分易于形成螺旋。
更新日期:2019-12-21
中文翻译:

α-突触核蛋白的构象偏差和瞬时结的形成。
我们通过使用具有显性和隐性溶剂的全原子模拟研究α-突触核蛋白动力学的局部构象偏倚。偏压与特定触点形成的频率有关。在这两种方法中,蛋白质都是内在无序的,其最强的偏见是使局部结构弯曲和转向。显式溶剂构象可以大大扩展,从而允许形成深和浅的短暂三叶形结,持续时间长达5μs。短寿命和长寿命的两链自缔合事件主要由序列中心部分的接触形成所决定。当与胶束结合时,该部分易于形成螺旋。











































 京公网安备 11010802027423号
京公网安备 11010802027423号