当前位置:
X-MOL 学术
›
Signal Transduct. Target Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DNA-PKcs promotes alcohol-related liver disease by activating Drp1-related mitochondrial fission and repressing FUNDC1-required mitophagy.
Signal Transduction and Targeted Therapy ( IF 40.8 ) Pub Date : 2019-12-06 , DOI: 10.1038/s41392-019-0094-1 Hao Zhou 1, 2 , Pingjun Zhu 1 , Jin Wang 1 , Sam Toan 3 , Jun Ren 2
Signal Transduction and Targeted Therapy ( IF 40.8 ) Pub Date : 2019-12-06 , DOI: 10.1038/s41392-019-0094-1 Hao Zhou 1, 2 , Pingjun Zhu 1 , Jin Wang 1 , Sam Toan 3 , Jun Ren 2
Affiliation
|
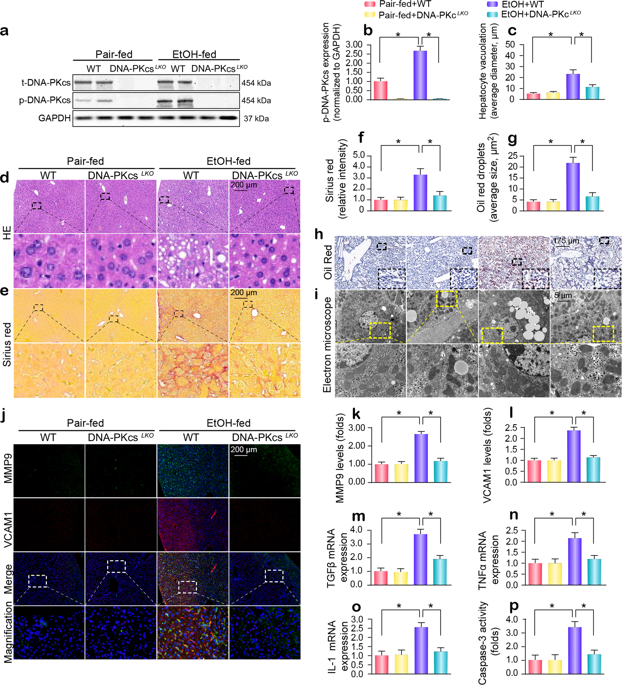
DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is a novel housekeeper of hepatic mitochondrial homeostasis outside the DNA repair process. In this study, DNA-PKcs was upregulated in the livers of mice that were exposed to alcohol; the expression of DNA-PKcs positively correlated with hepatic steatosis, fibrosis, apoptosis, and mitochondrial damage. Functional studies revealed that liver-specific DNA-PKcs knockout (DNA-PKcs LKO ) mice were protected from chronic ethanol-induced liver injury and mitochondrial damage. Mechanistic investigations established that DNA-PKcs promoted p53 activation, which elevated dynamin-related protein 1 (Drp1)-related mitochondrial fission but repressed FUN14 domain containing 1 (FUNDC1)-required mitophagy. Excessive fission and defective mitophagy triggered mtDNA damage, mitochondrial respiratory inhibition, mROS overproduction, cardiolipin oxidation, redox imbalance, calcium overload, and hepatic mitochondrial apoptosis. In contrast, the deletion of DNA-PKcs rescued these phenotypic alterations, which alleviated the susceptibility of hepatocytes to alcohol-induced cytotoxicity. Additionally, we also showed that orphan nuclear receptor subfamily 4 group A member 1 (NR4A1) was the upstream signal for DNA-PKcs activation and that the genetic ablation of NR4A1 ameliorated the progression of alcohol-related liver disease (ARLD); these results were similar to those obtained in DNA-PKcs knockout mice. Collectively, our results identified the NR4A1/DNA-PKcs/p53 axis as a novel signaling pathway responsible for ARLD pathogenesis that acts by activating Drp1-related mitochondrial fission and restricting FUNDC1-required mitophagy. The findings have potential implications for new approaches for ARLD therapy.
中文翻译:

DNA依赖性蛋白激酶催化亚基(DNA-PKcs)是DNA修复过程之外肝线粒体稳态的新型管家。在这项研究中,暴露于酒精的小鼠肝脏中 DNA-PKcs 上调;DNA-PKcs的表达与肝脂肪变性、纤维化、细胞凋亡和线粒体损伤呈正相关。功能研究表明,肝脏特异性 DNA-PKcs 敲除 (DNA-PKcs LKO) 小鼠可以免受慢性乙醇诱导的肝损伤和线粒体损伤。机制研究表明,DNA-PKcs 促进 p53 激活,从而提高动力相关蛋白 1 (Drp1) 相关的线粒体裂变,但抑制包含 FUN14 结构域 1 (FUNDC1) 所需的线粒体自噬。过度裂变和有缺陷的线粒体自噬引发线粒体DNA损伤、线粒体呼吸抑制、mROS过量产生、心磷脂氧化、氧化还原失衡、钙超载和肝线粒体凋亡。相反,DNA-PKcs 的删除挽救了这些表型改变,从而减轻了肝细胞对酒精诱导的细胞毒性的敏感性。此外,我们还发现孤儿核受体亚家族 4 A 组成员 1 (NR4A1) 是 DNA-PKcs 激活的上游信号,并且 NR4A1 的基因消除可改善酒精相关性肝病 (ARLD) 的进展;这些结果与 DNA-PKcs 敲除小鼠中获得的结果相似。总的来说,我们的结果确定 NR4A1/DNA-PKcs/p53 轴是一种负责 ARLD 发病机制的新型信号通路,通过激活 Drp1 相关的线粒体裂变和限制 FUNDC1 所需的线粒体自噬来发挥作用。这些发现对 ARLD 治疗的新方法具有潜在影响。
更新日期:2019-12-06
中文翻译:

DNA依赖性蛋白激酶催化亚基(DNA-PKcs)是DNA修复过程之外肝线粒体稳态的新型管家。在这项研究中,暴露于酒精的小鼠肝脏中 DNA-PKcs 上调;DNA-PKcs的表达与肝脂肪变性、纤维化、细胞凋亡和线粒体损伤呈正相关。功能研究表明,肝脏特异性 DNA-PKcs 敲除 (DNA-PKcs LKO) 小鼠可以免受慢性乙醇诱导的肝损伤和线粒体损伤。机制研究表明,DNA-PKcs 促进 p53 激活,从而提高动力相关蛋白 1 (Drp1) 相关的线粒体裂变,但抑制包含 FUN14 结构域 1 (FUNDC1) 所需的线粒体自噬。过度裂变和有缺陷的线粒体自噬引发线粒体DNA损伤、线粒体呼吸抑制、mROS过量产生、心磷脂氧化、氧化还原失衡、钙超载和肝线粒体凋亡。相反,DNA-PKcs 的删除挽救了这些表型改变,从而减轻了肝细胞对酒精诱导的细胞毒性的敏感性。此外,我们还发现孤儿核受体亚家族 4 A 组成员 1 (NR4A1) 是 DNA-PKcs 激活的上游信号,并且 NR4A1 的基因消除可改善酒精相关性肝病 (ARLD) 的进展;这些结果与 DNA-PKcs 敲除小鼠中获得的结果相似。总的来说,我们的结果确定 NR4A1/DNA-PKcs/p53 轴是一种负责 ARLD 发病机制的新型信号通路,通过激活 Drp1 相关的线粒体裂变和限制 FUNDC1 所需的线粒体自噬来发挥作用。这些发现对 ARLD 治疗的新方法具有潜在影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号