当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and photoinduced processes in molecular interlocked photosynthetic [60]fullerene systems.
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9cs00638a Jackson D Megiatto 1 , Dirk M Guldi , David I Schuster
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9cs00638a Jackson D Megiatto 1 , Dirk M Guldi , David I Schuster
Affiliation
|
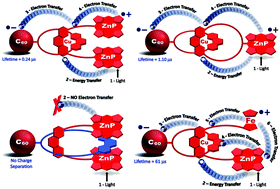
In natural photosynthesis, the protein backbone directs and positions primary and secondary electron donor and acceptor moieties in the reaction center to control the yield and kinetics of the sequential electron transfer reactions that transform light energy into chemical potential. Organization of the active cofactors is mainly driven by noncovalent interactions between the protein scaffold and the chromophores. Based on the natural system blueprint, several research efforts have investigated π-π stacking, ionic interactions as well as formation of hydrogen and coordinative bonds as noncovalent organizing principles for the assembly of electron donors and acceptors in artificial reaction centers. Introduction of supramolecular concepts into the organization of electron donor-acceptor in artificial photosynthetic models raises the possibility of applying template-directed synthesis techniques to assemble interlocked systems in which the photo-redox components are mechanically rather than covalently linked. Rotaxanes and catenanes are the leading examples of interlocked molecules, whose recent developments in synthetic chemistry have allowed their efficient preparation. Introduction of mechanical bonds into electron donor-acceptor systems allows the study of the interlocked components' submolecular motions and conformational changes, which are triggered by external stimuli, on the thermodynamic and kinetic parameters of photoinduced processes in artificial reaction centers. This Tutorial discusses our efforts in the synthesis and photophysical investigation of rotaxanes and catenanes decorated with peripheral electron donors and [60]fullerene as the acceptor. The assembly of our rotaxanes and catenanes is based on the classic 1,10-phenanthroline-copper(i) metal template strategy in conjunction with the virtues of the Cu(i)-catalyzed-1,3-dipolar cycloaddition of azides and alkynes (the CuAAC or "click" reaction) as the protocol for the final macrocyclization or stoppering reactions of the entwined precursors. Time-resolved emission and transient absorption experiments revealed that upon excitation, our multichromophoric rotaxanes and catenanes undergo a cascade of sequential energy and electron transfer reactions that ultimately yield charge separated states with lifetimes as long as 61 microseconds, thereby mimicking the functions of the natural systems. The importance of the Cu(i) ion (used to assemble the interlocked molecules) as an electronic relay in the photoinduced processes is also highlighted. The results of this research demonstrate the importance of the distinct molecular conformations adopted by rotaxanes and catenanes in the electron transfer dynamics and illustrate the versatility of interlocked molecules as scaffolds for the organization of donor-acceptor moieties in the design of artificial photosynthetic reaction centers.
中文翻译:

分子互锁的光合[60]富勒烯体系中的设计,合成和光诱导过程。
在自然光合作用中,蛋白质主链在反应中心指导和定位伯,次级电子供体和受体部分,以控制将光能转化为化学势的顺序电子转移反应的产率和动力学。活性辅因子的组织主要由蛋白质支架和发色团之间的非共价相互作用驱动。基于自然系统蓝图,数项研究工作已研究了π-π堆积,离子相互作用以及氢和配位键的形成,作为在人工反应中心组装电子供体和受体的非共价组织原理。在人工光合作用模型中将超分子概念引入电子供体-受体的组织中,提出了采用模板指导的合成技术来组装互锁系统的可能性,在互锁系统中,光氧化还原组分是机械而非共价连接的。轮烷和链烷是互锁分子的主要例子,它们在合成化学中的最新发展使得它们可以高效制备。将机械键引入电子给体-受体系统可以研究互锁组件的亚分子运动和构象变化,这些运动和构象变化是由外部刺激触发的,涉及人工反应中心中光诱导过程的热力学和动力学参数。本教程讨论了我们在用外围电子给体和[60]富勒烯作为受体修饰的轮烷和链烷的合成和光物理研究中所做的工作。我们的轮烷和链烷烃的组装基于经典的1,10-菲咯啉-铜(i)金属模板策略,并结合了铜(i)催化的叠氮化物和炔烃的1,,3-偶极环加成反应( CuAAC或“点击”反应)作为纠缠在一起的前体的最终大环化或终止反应的方案。时间分辨的发射和瞬态吸收实验表明,激发后,我们的多色轮烷和链烷经历了一系列连续的能量和电子转移反应,最终产生了电荷分离态,寿命长达61微秒,从而模仿自然系统的功能。还强调了Cu(i)离子(用于组装互锁分子)在光诱导过程中作为电子中继的重要性。这项研究的结果证明了轮烷和链烷所采用的独特分子构象在电子转移动力学中的重要性,并阐明了互锁分子作为支架的多功能性,从而可以在人工光合作用反应中心的设计中组织供体-受体部分。
更新日期:2020-01-02
中文翻译:

分子互锁的光合[60]富勒烯体系中的设计,合成和光诱导过程。
在自然光合作用中,蛋白质主链在反应中心指导和定位伯,次级电子供体和受体部分,以控制将光能转化为化学势的顺序电子转移反应的产率和动力学。活性辅因子的组织主要由蛋白质支架和发色团之间的非共价相互作用驱动。基于自然系统蓝图,数项研究工作已研究了π-π堆积,离子相互作用以及氢和配位键的形成,作为在人工反应中心组装电子供体和受体的非共价组织原理。在人工光合作用模型中将超分子概念引入电子供体-受体的组织中,提出了采用模板指导的合成技术来组装互锁系统的可能性,在互锁系统中,光氧化还原组分是机械而非共价连接的。轮烷和链烷是互锁分子的主要例子,它们在合成化学中的最新发展使得它们可以高效制备。将机械键引入电子给体-受体系统可以研究互锁组件的亚分子运动和构象变化,这些运动和构象变化是由外部刺激触发的,涉及人工反应中心中光诱导过程的热力学和动力学参数。本教程讨论了我们在用外围电子给体和[60]富勒烯作为受体修饰的轮烷和链烷的合成和光物理研究中所做的工作。我们的轮烷和链烷烃的组装基于经典的1,10-菲咯啉-铜(i)金属模板策略,并结合了铜(i)催化的叠氮化物和炔烃的1,,3-偶极环加成反应( CuAAC或“点击”反应)作为纠缠在一起的前体的最终大环化或终止反应的方案。时间分辨的发射和瞬态吸收实验表明,激发后,我们的多色轮烷和链烷经历了一系列连续的能量和电子转移反应,最终产生了电荷分离态,寿命长达61微秒,从而模仿自然系统的功能。还强调了Cu(i)离子(用于组装互锁分子)在光诱导过程中作为电子中继的重要性。这项研究的结果证明了轮烷和链烷所采用的独特分子构象在电子转移动力学中的重要性,并阐明了互锁分子作为支架的多功能性,从而可以在人工光合作用反应中心的设计中组织供体-受体部分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号