Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-12-05 , DOI: 10.1016/s0140-6736(19)32971-x Atul Deodhar 1 , Désirée van der Heijde 2 , Lianne S Gensler 3 , Tae-Hwan Kim 4 , Walter P Maksymowych 5 , Mikkel Østergaard 6 , Denis Poddubnyy 7 , Helena Marzo-Ortega 8 , Louis Bessette 9 , Tetsuya Tomita 10 , Ann Leung 11 , Maja Hojnik 12 , Gaia Gallo 12 , Xiaoqi Li 12 , David Adams 12 , Hilde Carlier 12 , Joachim Sieper 7 ,
The Lancet ( IF 98.4 ) Pub Date : 2019-12-05 , DOI: 10.1016/s0140-6736(19)32971-x Atul Deodhar 1 , Désirée van der Heijde 2 , Lianne S Gensler 3 , Tae-Hwan Kim 4 , Walter P Maksymowych 5 , Mikkel Østergaard 6 , Denis Poddubnyy 7 , Helena Marzo-Ortega 8 , Louis Bessette 9 , Tetsuya Tomita 10 , Ann Leung 11 , Maja Hojnik 12 , Gaia Gallo 12 , Xiaoqi Li 12 , David Adams 12 , Hilde Carlier 12 , Joachim Sieper 7 ,
Affiliation
|
BACKGROUND
Ixekizumab, a high-affinity interleukin-17A (IL-17A) monoclonal antibody, has previously shown efficacy in radiographic axial spondyloarthritis (also known as ankylosing spondylitis). We aimed to evaluate the efficacy and safety of ixekizumab, an IL-17 inhibitor, in non-radiographic axial spondyloarthritis. Here, we report the primary results of COAST-X.
METHODS
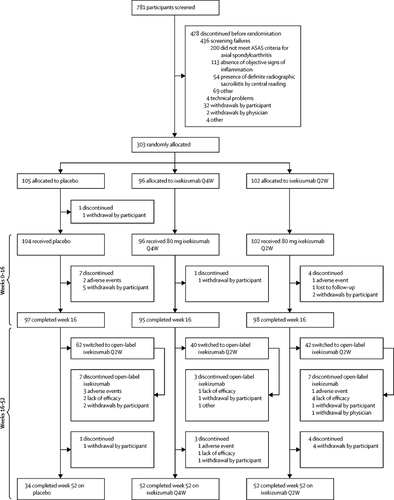
COAST-X was a 52-week, randomised, double-blind, placebo-controlled, parallel-group study done at 107 sites in 15 countries in Europe, Asia, North America, and South America. Eligible participants were adults (aged ≥18 years) with active axial spondyloarthritis without definite radiographic sacroiliitis (non-radiographic axial spondyloarthritis), objective signs of inflammation (via MRI or C-reactive protein), and an inadequate response or intolerance to non-steroidal anti-inflammatory drugs (NSAIDs). Patients were randomly assigned (1:1:1) to receive subcutaneous 80 mg ixekizumab every 4 weeks (Q4W) or every 2 weeks (Q2W), or placebo. Changing background medications or switching to open-label ixekizumab Q2W, or both, was allowed after week 16 at investigator discretion. Primary endpoints were Assessment of SpondyloArthritis international Society-40 (ASAS40) response (defined as an improvement of 40% or more and an absolute improvement from baseline of 2 units or more [range 0-10] in at least three of the four domains [patient global, spinal pain, function, and inflammation] without any worsening in the remaining one domain) at weeks 16 and 52. Patients who switched to open-label ixekizumab were imputed as non-responders in logistic regression analysis. This trial is registered with ClinicalTrials.gov, number NCT02757352.
FINDINGS
Between Aug 2, 2016, and Jan 29, 2018, 303 patients were enrolled (105 to placebo, 96 to ixekizumab Q4W, and 102 to ixekizumab Q2W). Both primary endpoints were met: ASAS40 at week 16 (ixekizumab Q4W: 34 [35%] of 96, p=0·0094 vs placebo; ixekizumab Q2W: 41 [40%] of 102, p=0·0016; placebo: 20 [19%] of 105) and ASAS40 at week 52 (ixekizumab Q4W: 29 [30%] of 96, p=0·0045; ixekizumab Q2W: 32 [31%] of 102, p=0·0037; placebo: 14 [13%] of 105). 60 (57%) of 104 patients in the placebo group, 63 (66%) of 96 in the ixekizumab Q4W group, and 79 (77%) of 102 in the ixekizumab Q2W group had at least one treatment-emergent adverse event. The most common treatment-emergent adverse events in the ixekizumab groups were nasopharyngitis and injection site reaction. Of the treatment-emergent adverse events of special interest, there was one case of serious infection in the ixekizumab Q4W group. The frequency of serious adverse events was low (four [1%] of 302) and similar across the three groups. There were no malignancies or deaths. No new safety signals were identified.
INTERPRETATION
Ixekizumab was superior to placebo for improving signs and symptoms in patients with non-radiographic axial spondyloarthritis at weeks 16 and 52. Reports of adverse events were similar to those of previous ixekizumab studies. Ixekizumab offers a potential therapeutic option for patients with non-radiographic axial spondyloarthritis who had an inadequate response or were intolerant to NSAID therapy.
FUNDING
Eli Lilly and Company.
中文翻译:

伊克珠单抗用于非放射性轴性脊柱关节炎(COAST-X)的患者:一项随机,安慰剂对照的试验。
背景技术伊克珠单抗,一种高亲和力的白介素17A(IL-17A)单克隆抗体,先前已显示出在放射照相的轴向脊椎关节炎(也称为强直性脊柱炎)中的功效。我们旨在评估IL-17抑制剂依克珠单抗在非放射线轴性脊柱关节炎中的疗效和安全性。在这里,我们报告COAST-X的主要结果。方法COAST-X是一项为期52周,随机,双盲,安慰剂对照,平行组的研究,研究在欧洲,亚洲,北美和南美的15个国家/地区的107个地点进行。符合条件的受试者为患有活动性轴颈脊椎炎的成人(≥18岁),而没有明确的放射学上的cro骨ili囊炎(非放射线型轴颈脊椎关节炎),炎症的客观体征(通过MRI或C反应蛋白),以及对非甾体类抗炎药(NSAID)的反应或耐受不良。患者被随机分配(1:1:1:1),每4周(Q4W)或每2周(Q2W)皮下注射80 mg依克珠单抗或安慰剂。在第16周后,研究人员可自行决定更换背景药物或改用开放标签的ixekizumab Q2W,或两者同时使用。主要终点为国际脊椎关节炎国际协会40(ASAS40)响应评估(定义为至少四个域中的三个域改善40%或更多,并且与基线相比绝对改善2个或更多单位[范围0-10] [在第16周和第52周,患者的整体,脊柱疼痛,功能和炎症),其余一个域没有任何恶化。切换到开放标签的ixekizumab的患者在logistic回归分析中被归为无反应者。该试验已在ClinicalTrials.gov上注册,编号为NCT02757352。结果在2016年8月2日至2018年1月29日之间,招募了303名患者(105名接受安慰剂,96名接受ixekizumab Q4W,102名接受ixekizumab Q2W)。达到两个主要终点:在第16周时达到ASAS40(依西珠单抗Q4W:96的34 [35%],p = 0·0094 vs安慰剂;依西珠单抗Q2W:102的41 [40%],p = 0·0016;安慰剂:20在第52周时[105]中的[19%]和ASAS40(依克珠单抗Q4W:96的29 [30%],p = 0·0045;依克珠单抗Q2W:102的32 [31%],p = 0·0037;安慰剂:14 [105%的[13%]。安慰剂组104例患者中有60例(57%),依克珠单抗Q4W组96例中有63例(66%),依西妥珠单抗Q2W组102例中有79例(77%)发生了至少一种治疗紧急不良事件。依克珠单抗组中最常见的治疗紧急事件是鼻咽炎和注射部位反应。在特别引起治疗的不良事件中,依克珠单抗Q4W组发生了1例严重感染。严重不良事件的发生率较低(在302个病例中,有四个[1%]),在三组中相似。没有恶性肿瘤或死亡。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。ixekizumab Q4W组中有1例严重感染。严重不良事件的发生率较低(在302个病例中,有四个[1%]),在三组中相似。没有恶性肿瘤或死亡。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。ixekizumab Q4W组中有1例严重感染。严重不良事件的发生率较低(在302个病例中,有四个[1%]),在三组中相似。没有恶性肿瘤或死亡。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为反应不充分或对NSAID治疗不耐受的非放射线轴性脊椎关节炎的患者提供了潜在的治疗选择。资金礼来公司。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。
更新日期:2020-01-04
中文翻译:

伊克珠单抗用于非放射性轴性脊柱关节炎(COAST-X)的患者:一项随机,安慰剂对照的试验。
背景技术伊克珠单抗,一种高亲和力的白介素17A(IL-17A)单克隆抗体,先前已显示出在放射照相的轴向脊椎关节炎(也称为强直性脊柱炎)中的功效。我们旨在评估IL-17抑制剂依克珠单抗在非放射线轴性脊柱关节炎中的疗效和安全性。在这里,我们报告COAST-X的主要结果。方法COAST-X是一项为期52周,随机,双盲,安慰剂对照,平行组的研究,研究在欧洲,亚洲,北美和南美的15个国家/地区的107个地点进行。符合条件的受试者为患有活动性轴颈脊椎炎的成人(≥18岁),而没有明确的放射学上的cro骨ili囊炎(非放射线型轴颈脊椎关节炎),炎症的客观体征(通过MRI或C反应蛋白),以及对非甾体类抗炎药(NSAID)的反应或耐受不良。患者被随机分配(1:1:1:1),每4周(Q4W)或每2周(Q2W)皮下注射80 mg依克珠单抗或安慰剂。在第16周后,研究人员可自行决定更换背景药物或改用开放标签的ixekizumab Q2W,或两者同时使用。主要终点为国际脊椎关节炎国际协会40(ASAS40)响应评估(定义为至少四个域中的三个域改善40%或更多,并且与基线相比绝对改善2个或更多单位[范围0-10] [在第16周和第52周,患者的整体,脊柱疼痛,功能和炎症),其余一个域没有任何恶化。切换到开放标签的ixekizumab的患者在logistic回归分析中被归为无反应者。该试验已在ClinicalTrials.gov上注册,编号为NCT02757352。结果在2016年8月2日至2018年1月29日之间,招募了303名患者(105名接受安慰剂,96名接受ixekizumab Q4W,102名接受ixekizumab Q2W)。达到两个主要终点:在第16周时达到ASAS40(依西珠单抗Q4W:96的34 [35%],p = 0·0094 vs安慰剂;依西珠单抗Q2W:102的41 [40%],p = 0·0016;安慰剂:20在第52周时[105]中的[19%]和ASAS40(依克珠单抗Q4W:96的29 [30%],p = 0·0045;依克珠单抗Q2W:102的32 [31%],p = 0·0037;安慰剂:14 [105%的[13%]。安慰剂组104例患者中有60例(57%),依克珠单抗Q4W组96例中有63例(66%),依西妥珠单抗Q2W组102例中有79例(77%)发生了至少一种治疗紧急不良事件。依克珠单抗组中最常见的治疗紧急事件是鼻咽炎和注射部位反应。在特别引起治疗的不良事件中,依克珠单抗Q4W组发生了1例严重感染。严重不良事件的发生率较低(在302个病例中,有四个[1%]),在三组中相似。没有恶性肿瘤或死亡。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。ixekizumab Q4W组中有1例严重感染。严重不良事件的发生率较低(在302个病例中,有四个[1%]),在三组中相似。没有恶性肿瘤或死亡。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。ixekizumab Q4W组中有1例严重感染。严重不良事件的发生率较低(在302个病例中,有四个[1%]),在三组中相似。没有恶性肿瘤或死亡。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。没有发现新的安全信号。解释在第16周和第52周,伊克珠单抗在改善非放射状轴性脊椎关节炎患者的体征和症状方面优于安慰剂。不良事件的报告与以前的伊克珠单抗研究相似。伊克珠单抗为反应不充分或对NSAID治疗不耐受的非放射线轴性脊椎关节炎的患者提供了潜在的治疗选择。资金礼来公司。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。伊克珠单抗为那些反应不足或对NSAID治疗不耐受的非放射线轴性脊柱关节炎的患者提供了一种潜在的治疗选择。资金礼来公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号