当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preferential Recruitment of Conformationally Distinct Amyloid-β Oligomers by the Intrinsically Disordered Region of the Human Prion Protein.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2019-12-20 , DOI: 10.1021/acschemneuro.9b00646 Priyanka Madhu , Samrat Mukhopadhyay
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2019-12-20 , DOI: 10.1021/acschemneuro.9b00646 Priyanka Madhu , Samrat Mukhopadhyay
|
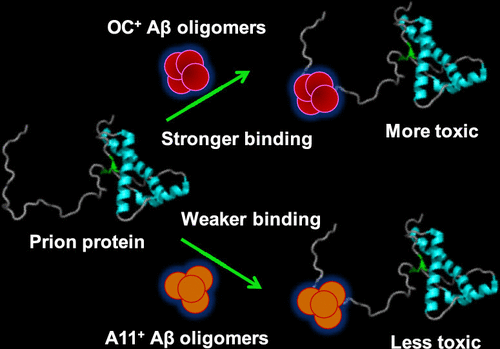
Soluble oligomeric species of the amyloid-β (Aβ) peptide exhibit pronounced neurotoxic effects in Alzheimer's disease. Recent studies have indicated that the prion protein (PrP) is one of the cell-surface receptors, so-called a bad receptor, of Aβ oligomers that mediates downstream cellular toxicity. A rational classification of Aβ oligomers on the basis of conformation indicates that there are two distinct types of oligomers, namely, prefibrillar and fibrillar oligomers that are positive to A11 and OC conformation-dependent antibodies, respectively. The mechanism of heterotypic assembly of conformationally distinct oligomers and PrP is poorly understood. In this work, using an array of biophysical and biochemical tools, we dissect the molecular mechanism of the interaction of A11- and OC-positive Aβ42 oligomers with human PrP. Using site-specific binding titrations, we show that the recruitment of Aβ oligomers primarily occurs via the electrostatic interaction between the N-terminal intrinsically disordered region of PrP and Aβ oligomers. Our results demonstrate that OC-positive fibrillar oligomers possessing in-register parallel β-sheet packing displayed ∼30 times stronger binding with PrP compared to A11-positive oligomers. We also show that these OC-positive oligomers exacerbate their toxic effects on mammalian cells upon binding to PrP. On the contrary, the addition of PrP does not alter the toxicity exhibited by A11-positive oligomers. Our findings suggest that strategies targeting the interaction between PrP and OC-positive oligomers, which have been shown to be highly concentrated in the vicinity of amyloid plaques, may have therapeutic potential against Alzheimer's disease.
中文翻译:

通过人类Pri病毒蛋白的内在无序区域优先募集构象不同的淀粉样β-寡聚体。
淀粉样β(Aβ)肽的可溶性寡聚体在阿尔茨海默氏病中表现出明显的神经毒性作用。最近的研究表明the病毒蛋白(PrP)是介导下游细胞毒性的Aβ低聚物的细胞表面受体之一,即所谓的坏受体。根据构象对Aβ低聚物进行合理分类,表明存在两种不同类型的低聚物,分别是对A11和OC构象依赖性抗体呈阳性的原纤维前和原纤维低聚物。构象不同的寡聚物和PrP的异型组装的机制了解甚少。在这项工作中,我们使用一系列生物物理和生化工具,剖析了A11和OC阳性Aβ42低聚物与人PrP相互作用的分子机制。使用位点特异性结合滴定,我们显示Aβ低聚物的募集主要是通过PrP的N端固有无序区域与Aβ低聚物之间的静电相互作用发生的。我们的结果表明,与A11阳性低聚物相比,具有寄存器内平行β-折叠堆积的OC阳性原纤维低聚物显示出与PrP的结合力强约30倍。我们还显示,这些OC阳性低聚物在结合PrP时会加剧其对哺乳动物细胞的毒性作用。相反,PrP的添加不会改变A11阳性低聚物所表现出的毒性。我们的发现表明,针对PrP和OC阳性低聚物之间相互作用的策略已被证明高度集中在淀粉样斑块附近,
更新日期:2019-12-21
中文翻译:

通过人类Pri病毒蛋白的内在无序区域优先募集构象不同的淀粉样β-寡聚体。
淀粉样β(Aβ)肽的可溶性寡聚体在阿尔茨海默氏病中表现出明显的神经毒性作用。最近的研究表明the病毒蛋白(PrP)是介导下游细胞毒性的Aβ低聚物的细胞表面受体之一,即所谓的坏受体。根据构象对Aβ低聚物进行合理分类,表明存在两种不同类型的低聚物,分别是对A11和OC构象依赖性抗体呈阳性的原纤维前和原纤维低聚物。构象不同的寡聚物和PrP的异型组装的机制了解甚少。在这项工作中,我们使用一系列生物物理和生化工具,剖析了A11和OC阳性Aβ42低聚物与人PrP相互作用的分子机制。使用位点特异性结合滴定,我们显示Aβ低聚物的募集主要是通过PrP的N端固有无序区域与Aβ低聚物之间的静电相互作用发生的。我们的结果表明,与A11阳性低聚物相比,具有寄存器内平行β-折叠堆积的OC阳性原纤维低聚物显示出与PrP的结合力强约30倍。我们还显示,这些OC阳性低聚物在结合PrP时会加剧其对哺乳动物细胞的毒性作用。相反,PrP的添加不会改变A11阳性低聚物所表现出的毒性。我们的发现表明,针对PrP和OC阳性低聚物之间相互作用的策略已被证明高度集中在淀粉样斑块附近,











































 京公网安备 11010802027423号
京公网安备 11010802027423号