当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-assembling as regular nanoparticles dramatically minimizes photobleaching of tumour-targeted GFP.
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2019-12-06 , DOI: 10.1016/j.actbio.2019.12.003 Ugutz Unzueta 1 , Mònica Roldán 2 , Mireia Pesarrodona 3 , Raul Benitez 4 , Alejandro Sánchez-Chardi 5 , Oscar Conchillo-Solé 6 , Ramón Mangues 7 , Antonio Villaverde 3 , Esther Vázquez 3
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2019-12-06 , DOI: 10.1016/j.actbio.2019.12.003 Ugutz Unzueta 1 , Mònica Roldán 2 , Mireia Pesarrodona 3 , Raul Benitez 4 , Alejandro Sánchez-Chardi 5 , Oscar Conchillo-Solé 6 , Ramón Mangues 7 , Antonio Villaverde 3 , Esther Vázquez 3
Affiliation
|
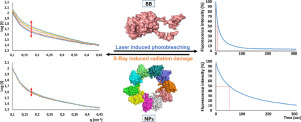
Fluorescent proteins are useful imaging and theranostic agents, but their potential superiority over alternative dyes is weakened by substantial photobleaching under irradiation. Enhancing protein photostability has been attempted through diverse strategies, with irregular results and limited applicability. In this context, we wondered if the controlled oligomerization of Green Fluorescent Protein (GFP) as nanoscale supramolecular complexes could stabilize the fluorophore through the newly formed protein-protein contacts, and thus, enhance its global photostability. For that, we have here analyzed the photobleaching profile of several GFP versions, engineered to self-assemble as tumour-homing nanoparticles with different targeting, size and structural stability. This has been done under prolonged irradiation in confocal laser scanning microscopy and by small-angle X-ray scattering. The results show that the oligomerization of GFP at the nanoscale enhances, by more than seven-fold, the stability of fluorescence emission. Interestingly, GFP nanoparticles are much more resistant to X-ray damage than the building block counterparts, indicating that the gained photostability is linked to enhanced structural resistance to radiation. Therefore, the controlled oligomerization of self-assembling fluorescent proteins as protein nanoparticles is a simple, versatile and powerful method to enhance their photostability for uses in precision imaging and therapy. STATEMENT OF SIGNIFICANCE: Fluorescent protein assembly into regular and highly symmetric nanoscale structures has been identified to confer enhanced structural stability against radiation stresses dramatically reducing their photobleaching. Being this the main bottleneck in the use of fluorescent proteins for imaging and theranostics, this protein architecture engineering principle appears as a powerful method to enhance their photostability for a broad applicability in precision imaging, drug delivery and theranostics.
中文翻译:

自组装成规则的纳米粒子可最大程度地减少肿瘤靶向GFP的光漂白。
荧光蛋白是有用的成像和诊断试剂,但是它们在替代染料下的潜在优势由于在辐射下大量的光漂白而减弱了。已经尝试通过多种策略来增强蛋白质的光稳定性,但结果不规则且适用性有限。在这种情况下,我们想知道绿色荧光蛋白(GFP)作为纳米级超分子复合物的受控寡聚化是否可以通过新形成的蛋白-蛋白接触来稳定荧光团,从而增强其整体光稳定性。为此,我们在这里分析了几种GFP版本的光漂白特性,这些GFP版本被设计成可自组装成具有不同靶向,大小和结构稳定性的肿瘤归巢纳米粒子。这是在共聚焦激光扫描显微镜中长时间照射下以及通过小角度X射线散射完成的。结果表明,在纳米尺度上,GFP的寡聚提高了七倍以上的荧光发射稳定性。有趣的是,GFP纳米颗粒对X射线损伤的抵抗力要比对等结构块更高,表明获得的光稳定性与增强的结构抗辐射能力有关。因此,自组装荧光蛋白作为蛋白纳米颗粒的受控寡聚化是一种简单,通用且功能强大的方法,可增强其光稳定性,用于精密成像和治疗。重要性声明:荧光蛋白组装成规则的和高度对称的纳米级结构已被鉴定为赋予增强的结构稳定性以抵抗辐射应力,从而大大减少了它们的光漂白。作为使用荧光蛋白进行成像和治疗诊断的主要瓶颈,这种蛋白质结构工程原理似乎是一种增强其光稳定性的有效方法,可广泛应用于精密成像,药物递送和治疗诊断。
更新日期:2019-12-06
中文翻译:

自组装成规则的纳米粒子可最大程度地减少肿瘤靶向GFP的光漂白。
荧光蛋白是有用的成像和诊断试剂,但是它们在替代染料下的潜在优势由于在辐射下大量的光漂白而减弱了。已经尝试通过多种策略来增强蛋白质的光稳定性,但结果不规则且适用性有限。在这种情况下,我们想知道绿色荧光蛋白(GFP)作为纳米级超分子复合物的受控寡聚化是否可以通过新形成的蛋白-蛋白接触来稳定荧光团,从而增强其整体光稳定性。为此,我们在这里分析了几种GFP版本的光漂白特性,这些GFP版本被设计成可自组装成具有不同靶向,大小和结构稳定性的肿瘤归巢纳米粒子。这是在共聚焦激光扫描显微镜中长时间照射下以及通过小角度X射线散射完成的。结果表明,在纳米尺度上,GFP的寡聚提高了七倍以上的荧光发射稳定性。有趣的是,GFP纳米颗粒对X射线损伤的抵抗力要比对等结构块更高,表明获得的光稳定性与增强的结构抗辐射能力有关。因此,自组装荧光蛋白作为蛋白纳米颗粒的受控寡聚化是一种简单,通用且功能强大的方法,可增强其光稳定性,用于精密成像和治疗。重要性声明:荧光蛋白组装成规则的和高度对称的纳米级结构已被鉴定为赋予增强的结构稳定性以抵抗辐射应力,从而大大减少了它们的光漂白。作为使用荧光蛋白进行成像和治疗诊断的主要瓶颈,这种蛋白质结构工程原理似乎是一种增强其光稳定性的有效方法,可广泛应用于精密成像,药物递送和治疗诊断。



























 京公网安备 11010802027423号
京公网安备 11010802027423号