当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile Synthesis of α‐Haloketones by Aerobic Oxidation of Olefins Using KX as Nonhazardous Halogen Source
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2019-12-24 , DOI: 10.1002/cjoc.201900372 Zhibin Luo 1 , Yunge Meng 1 , Xinchi Gong 1 , Jie Wu 1 , Yulan Zhang 1 , Long‐Wu Ye 2 , Chunyin Zhu 1, 2
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2019-12-24 , DOI: 10.1002/cjoc.201900372 Zhibin Luo 1 , Yunge Meng 1 , Xinchi Gong 1 , Jie Wu 1 , Yulan Zhang 1 , Long‐Wu Ye 2 , Chunyin Zhu 1, 2
Affiliation
|
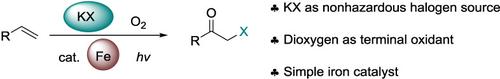
An operationally simple and safe synthesis of α‐haloketones using KBr and KCl as nonhazardous halogen sources is reported. It involves an iron‐catalysed reaction of alkenes with KBr/KCl using O2 as terminal oxidant under the irradiation of visible‐light. This strategy avoids the risks associated with handling halo‐contained electrophiles (Cl2, Br2, NCS, NBS). The process is tolerant to several functional groups, and extended to a range of substituted styrenes in up to 89% yield. A radical reaction pathway is proposed based on control experiments and spectroscopy studies.
中文翻译:

以KX为无害卤素源的烯烃好氧氧化法轻松合成α-卤代酮
据报道,使用KBr和KCl作为无害卤素源,可以简单,安全地合成α-卤代酮。它涉及在可见光照射下,以O 2为末端氧化剂,烯烃与KBr / KCl的铁催化反应。该策略避免了与处理含卤亲电试剂(Cl 2,Br 2,NCS,NBS)相关的风险。该方法可耐受多个官能团,并以高达89%的收率扩展到一系列取代苯乙烯。基于控制实验和光谱学研究,提出了自由基反应途径。
更新日期:2019-12-25
中文翻译:

以KX为无害卤素源的烯烃好氧氧化法轻松合成α-卤代酮
据报道,使用KBr和KCl作为无害卤素源,可以简单,安全地合成α-卤代酮。它涉及在可见光照射下,以O 2为末端氧化剂,烯烃与KBr / KCl的铁催化反应。该策略避免了与处理含卤亲电试剂(Cl 2,Br 2,NCS,NBS)相关的风险。该方法可耐受多个官能团,并以高达89%的收率扩展到一系列取代苯乙烯。基于控制实验和光谱学研究,提出了自由基反应途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号