当前位置:
X-MOL 学术
›
Chin. J. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced photocatalytic hydrogen production activity of highly crystalline carbon nitride synthesized by hydrochloric acid treatment
Chinese Journal of Catalysis ( IF 15.7 ) Pub Date : 2020-01-01 , DOI: 10.1016/s1872-2067(19)63427-3 Yang Li , Dainan Zhang , Xionghan Feng , Quanjun Xiang
Chinese Journal of Catalysis ( IF 15.7 ) Pub Date : 2020-01-01 , DOI: 10.1016/s1872-2067(19)63427-3 Yang Li , Dainan Zhang , Xionghan Feng , Quanjun Xiang
|
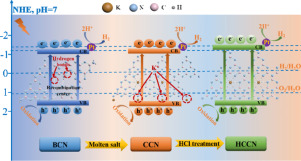
Abstract Crystalline carbon nitride (CCN) prepared by a molten-salt method is attracting increased attention because of its promising properties and excellent photocatalytic activity. In this work, we further improve the crystallinity of CCN through synthesis by the molten-salt method under the action of aqueous hydrochloric acid (HCl) solution. Our results showed that the cryst allinity of the as-prepared samples increased with increasing HCl concentration and reached the maximum value at 0.1 mol L−1. This can be attributed to the removal of some potassium ions (K+) from the terminal amino groups of CCN by the aqueous HCl solution, which results in a release of the polymerization sites. As a result, the crystallinity of the as-prepared samples further increased. Moreover, the obtained 0.1 highly crystalline carbon nitride (0.1HCCN; treated with 0.1 mol L−1 aqueous HCl solution) exhibited an excellent photocatalytic hydrogen evolution of 683.54 µmol h−1 g−1 and a quantum efficiency of 6.6% at 420 nm with triethanolamine as the sacrificial agent. This photocatalytic hydrogen evolution was 2 and 10 times higher than those of CCN and bulk carbon nitride, respectively. The enhanced photocatalytic activity was attributed to the improved crystallinity and intercalation of K+ into the xHCCN interlayer. The improved crystallinity can decrease the number of surface defects and hydrogen bonds in the as-prepared sample, thereby increasing the mobility of the photoinduced carriers and reducing the recombination sites of the electron–hole pairs. The K+ intercalated into the xHCCN interlayer also promoted the transfer of the photoinduced electrons because these ions can increase the electronic delocalization and extend the π-conjugated systems. This study may provide new insights into the further development of the molten-salt method.
中文翻译:

盐酸处理合成的高结晶氮化碳的光催化制氢活性增强
摘要 熔盐法制备的结晶氮化碳(CCN)因其良好的性能和优异的光催化活性而受到越来越多的关注。在这项工作中,我们通过在盐酸 (HCl) 水溶液的作用下通过熔盐法合成来进一步提高 CCN 的结晶度。我们的结果表明,所制备样品的结晶度随着 HCl 浓度的增加而增加,并在 0.1 mol L−1 时达到最大值。这可能是由于 HCl 水溶液从 CCN 的末端氨基中去除了一些钾离子 (K+),导致聚合位点的释放。结果,所制备样品的结晶度进一步增加。此外,所得0.1高结晶氮化碳(0.1HCCN;用0. 1 mol L-1 HCl 水溶液)表现出优异的光催化析氢量 683.54 µmol h-1 g-1,在 420 nm 下以三乙醇胺为牺牲剂的量子效率为 6.6%。这种光催化析氢分别比 CCN 和块状氮化碳高 2 倍和 10 倍。增强的光催化活性归因于提高的结晶度和 K+ 嵌入 xHCCN 夹层中。提高的结晶度可以减少所制备样品中的表面缺陷和氢键的数量,从而增加光生载流子的迁移率并减少电子 - 空穴对的复合位点。插入到 xHCCN 夹层中的 K+ 也促进了光生电子的转移,因为这些离子可以增加电子离域并扩展 π 共轭系统。这项研究可能为熔盐法的进一步发展提供新的见解。
更新日期:2020-01-01
中文翻译:

盐酸处理合成的高结晶氮化碳的光催化制氢活性增强
摘要 熔盐法制备的结晶氮化碳(CCN)因其良好的性能和优异的光催化活性而受到越来越多的关注。在这项工作中,我们通过在盐酸 (HCl) 水溶液的作用下通过熔盐法合成来进一步提高 CCN 的结晶度。我们的结果表明,所制备样品的结晶度随着 HCl 浓度的增加而增加,并在 0.1 mol L−1 时达到最大值。这可能是由于 HCl 水溶液从 CCN 的末端氨基中去除了一些钾离子 (K+),导致聚合位点的释放。结果,所制备样品的结晶度进一步增加。此外,所得0.1高结晶氮化碳(0.1HCCN;用0. 1 mol L-1 HCl 水溶液)表现出优异的光催化析氢量 683.54 µmol h-1 g-1,在 420 nm 下以三乙醇胺为牺牲剂的量子效率为 6.6%。这种光催化析氢分别比 CCN 和块状氮化碳高 2 倍和 10 倍。增强的光催化活性归因于提高的结晶度和 K+ 嵌入 xHCCN 夹层中。提高的结晶度可以减少所制备样品中的表面缺陷和氢键的数量,从而增加光生载流子的迁移率并减少电子 - 空穴对的复合位点。插入到 xHCCN 夹层中的 K+ 也促进了光生电子的转移,因为这些离子可以增加电子离域并扩展 π 共轭系统。这项研究可能为熔盐法的进一步发展提供新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号