当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic phase equilibria in the aqueous ternary system NaCl–NaBO2–H2O: experimental data and solubility calculation using the Pitzer model
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jct.2019.106021 Lan Yang , Dan Li , Tao Zhang , Lingzong Meng , Tianlong Deng , Yafei Guo
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jct.2019.106021 Lan Yang , Dan Li , Tao Zhang , Lingzong Meng , Tianlong Deng , Yafei Guo
|
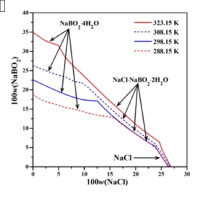
Abstract Solubilities and refractive indices in the ternary system NaCl–NaBO2–H2O at 323.15 K were investigated with the dissolution equilibrium method. The phase diagram of this system consists of three single-salt crystallization regions for NaBO2·4H2O, NaCl·NaBO2·2H2O and NaCl, three univariant solubility curves and two invariant points. A comparison of the diagrams of this system from 288.15 to 323.15 K shows that area of NaBO2·4H2O region decreased whereas the areas of NaCl·NaBO2·2H2O increased obviously with temperature increasing. The Pitzer binary parameters of NaB(OH)4, Pitzer mixing parameters θ Cl - , B OH 4 - and ψ Na + , Cl - , B OH 4 - , and the dissolution equilibrium constants of NaB(OH)4·2H2O and NaCl·NaB(OH)4 were fitted with the solubility data on the basis of Pitzer model. The variation trends for calculated solubility curves are in accordance with the experimental results. The results show that the other boron species except B(OH)4− in the solution for the system NaCl–NaB(OH)4–H2O can’t be neglected. The assumption of only one boron containing ionic species B(OH)4− in the solution is not very accurate, but the calculated results with the assumption can still describe the experimental values. The Pitzer parameters and Ksp values obtained in this study are capable of solubility calculation for the system containing B(OH)4−.
中文翻译:

NaCl–NaBO2–H2O 三元水溶液中的热力学相平衡:实验数据和使用 Pitzer 模型的溶解度计算
摘要 采用溶解平衡法研究了 NaCl-NaBO2-H2O 在 323.15 K 三元体系中的溶解度和折光率。该体系的相图由NaBO2·4H2O、NaCl·NaBO2·2H2O和NaCl的三个单盐结晶区、三个单变溶解度曲线和两个不变点组成。比较该体系在288.15~323.15 K的图谱,随着温度的升高,NaBO2·4H2O区域的面积减小,而NaCl·NaBO2·2H2O的面积明显增加。NaB(OH)4的Pitzer二元参数、Pitzer混合参数θ Cl - 、B OH 4 - 和ψ Na + 、Cl - 、B OH 4 - 以及NaB(OH)4·2H2O和NaCl的溶解平衡常数·NaB(OH)4根据Pitzer模型拟合溶解度数据。计算出的溶解度曲线的变化趋势与实验结果一致。结果表明,NaCl–NaB(OH)4–H2O 体系溶液中除B(OH)4− 外的其他硼物种均不可忽视。溶液中只有一种含硼离子物质 B(OH)4- 的假设不是很准确,但该假设的计算结果仍然可以描述实验值。本研究中获得的 Pitzer 参数和 Ksp 值能够计算含有 B(OH)4− 的系统的溶解度。但假设的计算结果仍然可以描述实验值。本研究中获得的 Pitzer 参数和 Ksp 值能够计算含有 B(OH)4− 的系统的溶解度。但假设的计算结果仍然可以描述实验值。本研究中获得的 Pitzer 参数和 Ksp 值能够计算含有 B(OH)4− 的系统的溶解度。
更新日期:2020-01-01
中文翻译:

NaCl–NaBO2–H2O 三元水溶液中的热力学相平衡:实验数据和使用 Pitzer 模型的溶解度计算
摘要 采用溶解平衡法研究了 NaCl-NaBO2-H2O 在 323.15 K 三元体系中的溶解度和折光率。该体系的相图由NaBO2·4H2O、NaCl·NaBO2·2H2O和NaCl的三个单盐结晶区、三个单变溶解度曲线和两个不变点组成。比较该体系在288.15~323.15 K的图谱,随着温度的升高,NaBO2·4H2O区域的面积减小,而NaCl·NaBO2·2H2O的面积明显增加。NaB(OH)4的Pitzer二元参数、Pitzer混合参数θ Cl - 、B OH 4 - 和ψ Na + 、Cl - 、B OH 4 - 以及NaB(OH)4·2H2O和NaCl的溶解平衡常数·NaB(OH)4根据Pitzer模型拟合溶解度数据。计算出的溶解度曲线的变化趋势与实验结果一致。结果表明,NaCl–NaB(OH)4–H2O 体系溶液中除B(OH)4− 外的其他硼物种均不可忽视。溶液中只有一种含硼离子物质 B(OH)4- 的假设不是很准确,但该假设的计算结果仍然可以描述实验值。本研究中获得的 Pitzer 参数和 Ksp 值能够计算含有 B(OH)4− 的系统的溶解度。但假设的计算结果仍然可以描述实验值。本研究中获得的 Pitzer 参数和 Ksp 值能够计算含有 B(OH)4− 的系统的溶解度。但假设的计算结果仍然可以描述实验值。本研究中获得的 Pitzer 参数和 Ksp 值能够计算含有 B(OH)4− 的系统的溶解度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号