当前位置:
X-MOL 学术
›
Lancet Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1-2 trial.
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-12-04 , DOI: 10.1016/s1470-2045(19)30671-0 Birgit Geoerger 1 , Hyoung Jin Kang 2 , Michal Yalon-Oren 3 , Lynley V Marshall 4 , Catherine Vezina 5 , Alberto Pappo 6 , Theodore W Laetsch 7 , Antonio S Petrilli 8 , Martin Ebinger 9 , Jacek Toporski 10 , Julia Glade-Bender 11 , Wayne Nicholls 12 , Elizabeth Fox 13 , Steven G DuBois 14 , Margaret E Macy 15 , Susan L Cohn 16 , Kumudu Pathiraja 17 , Scott J Diede 17 , Scot Ebbinghaus 17 , Navin Pinto 18
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-12-04 , DOI: 10.1016/s1470-2045(19)30671-0 Birgit Geoerger 1 , Hyoung Jin Kang 2 , Michal Yalon-Oren 3 , Lynley V Marshall 4 , Catherine Vezina 5 , Alberto Pappo 6 , Theodore W Laetsch 7 , Antonio S Petrilli 8 , Martin Ebinger 9 , Jacek Toporski 10 , Julia Glade-Bender 11 , Wayne Nicholls 12 , Elizabeth Fox 13 , Steven G DuBois 14 , Margaret E Macy 15 , Susan L Cohn 16 , Kumudu Pathiraja 17 , Scott J Diede 17 , Scot Ebbinghaus 17 , Navin Pinto 18
Affiliation
|
BACKGROUND
Pembrolizumab is approved for the treatment of advanced cancer in adults; however, no information is available on safety and efficacy in paediatric patients. We aimed to establish the recommended phase 2 dose of pembrolizumab and its safety and antitumour activity in advanced paediatric cancer.
METHODS
KEYNOTE-051 is an ongoing phase 1-2 open-label trial. In this interim analysis, children aged 6 months to 17 years were recruited at 30 hospitals located in Australia, Brazil, Canada, France, Germany, Israel, Italy, South Korea, Sweden, the UK, and the USA. Patients with melanoma or a centrally confirmed, PD-L1-positive, relapsed or refractory solid tumour or lymphoma, and a Lansky Play/Karnofsky Performance status score of 50 or higher, received intravenous pembrolizumab at an initial dose of 2 mg/kg every 3 weeks. Pharmacokinetics and dose-limiting toxicities were used to establish the recommended phase 2 dose, and the safety and antitumour activity of this dose were assessed. Primary endpoints were determination of dose-limiting toxicities at the maximum administered dose, safety and tolerability, and the proportion of patients with objective response to pembrolizumab for each tumour type according to the Response Evaluation Criteria in Solid Tumours version 1.1 or the International Neuroblastoma Response Criteria. Safety and efficacy were assessed in all treated patients who received at least one dose of pembrolizumab. Separate reporting of the cohort of patients with relapsed or refractory classical Hodgkin lymphoma was a post-hoc decision. The data cutoff for this interim analysis was Sept 3, 2018. This trial is still enrolling patients and is registered with ClinicalTrials.gov, number NCT02332668.
FINDINGS
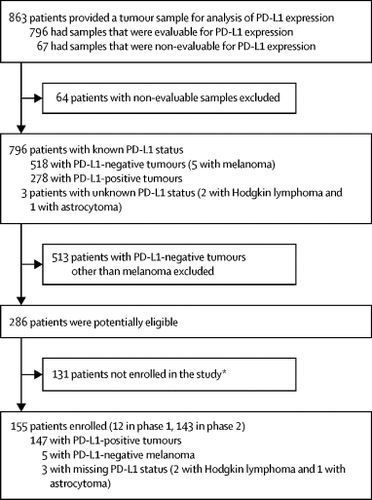
Of 863 patients screened between March 23, 2015, and Sept 3, 2018, 796 had tumours that were evaluable for PD-L1 expression (278 [35%] were PD-L1-positive); 155 eligible patients were enrolled and 154 had at least one dose of pembrolizumab. The median age of the enrolled patients was 13 years (IQR 8-15). Median follow-up was 8·6 months (IQR 2·5-16·4). No dose-limiting toxicities were reported in phase 1, and pembrolizumab plasma concentrations were consistent with those previously reported in adults; the recommended phase 2 dose was therefore established as 2 mg/kg every 3 weeks. Of the 154 patients treated, 69 (45%) experienced grade 3-5 adverse events, most commonly anaemia in 14 (9%) patients and decreased lymphocyte count in nine (6%) patients. 13 (8%) of the 154 patients had grade 3-5 treatment-related adverse events, most commonly decreased lymphocyte count in three (2%) patients and anaemia in two (1%) patients. 14 (9%) patients had serious treatment-related adverse events, most commonly pyrexia (four [3%]), and hypertension and pleural effusion (two [1%] each). Four patients (3%) discontinued treatment because of treatment-related adverse events, and two (1%) died (one due to pulmonary oedema and one due to pleural effusion and pneumonitis). Of 15 patients with relapsed or refractory Hodgkin lymphoma, two had complete and seven had partial responses; thus, nine patients achieved an objective response (60·0%; 95% CI 32·3-83·7). Of 136 patients with solid tumours and other lymphomas, eight had partial responses (two patients each with adrenocortical carcinoma and mesothelioma, and one patient each with malignant ganglioglioma, epithelioid sarcoma, lymphoepithelial carcinoma, and malignant rhabdoid tumour); the proportion of patients with an objective response was 5·9% (95% CI 2·6-11·3).
INTERPRETATION
Pembrolizumab was well tolerated and showed encouraging antitumour activity in paediatric patients with relapsed or refractory Hodgkin lymphoma, consistent with experience in adult patients. Pembrolizumab had low antitumour activity in the majority of paediatric tumour types, and responses were observed in only a few rare PD-L1-positive tumour types, suggesting that PD-L1 expression alone is not sufficient as a biomarker for the selection of paediatric patients who are likely to respond to PD-1 checkpoint inhibitors. Final results of KEYNOTE-051, expected by September, 2022, with the possibility for extension, will report further on the activity of pembrolizumab in Hodgkin lymphoma, microsatellite instability-high tumours, and melanoma.
FUNDING
Merck Sharp & Dohme, a subsidiary of Merck & Co.
中文翻译:

Pembrolizumab在患有晚期黑素瘤或PD-L1阳性,晚期,复发或难治性实体瘤或淋巴瘤的小儿患者中(KEYNOTE-051):一项开放性单臂1-2期试验的中期分析。
背景技术派姆单抗被批准用于治疗成人晚期癌症。但是,尚无有关儿科患者安全性和有效性的信息。我们的目标是确定推荐的2期剂量的派姆单抗及其在晚期小儿癌症中的安全性和抗肿瘤活性。方法KEYNOTE-051是一项正在进行的1-2期开放标签试验。在此中期分析中,从位于澳大利亚,巴西,加拿大,法国,德国,以色列,意大利,韩国,瑞典,英国和美国的30家医院中招募了6个月至17岁的儿童。黑色素瘤或中心确认的PD-L1阳性,复发或难治性实体瘤或淋巴瘤且Lansky Play / Karnofsky Performance状态评分为50或更高的患者,每3剂接受静脉注射pembrolizumab,初始剂量为2 mg / kg周。使用药代动力学和剂量限制性毒性确定推荐的2期剂量,并评估该剂量的安全性和抗肿瘤活性。主要终点是根据实体肿瘤1.1版或国际神经母细胞瘤反应标准中的反应评价标准,确定最大给药剂量,安全性和耐受性下的剂量限制性毒性以及每种肿瘤对pembrolizumab客观反应的患者比例。在接受至少一剂派姆单抗治疗的所有接受治疗的患者中评估安全性和有效性。复发或难治性经典霍奇金淋巴瘤患者队列的单独报告是事后决定。此中期分析的数据截止日期为2018年9月3日。该试验仍在招募患者,并已在ClinicalTrials.gov上注册,编号为NCT02332668。在2015年3月23日至2018年9月3日之间筛查的863例患者中,有796例可评估PD-L1表达的肿瘤(278例[35%]为PD-L1阳性); 纳入155位合格患者,其中154位患者接受至少一剂派姆单抗治疗。入组患者的中位年龄为13岁(IQR 8-15)。中位随访时间为8·6个月(IQR 2·5-16·4)。在第1阶段没有报道剂量限制性毒性,并且派姆单抗的血浆浓度与以前在成人中报道的浓度一致;因此,建议的第2阶段推荐剂量为每3周2 mg / kg。在接受治疗的154位患者中,有69位(占45%)经历了3-5级不良事件,最常见的是贫血,有14位(9%)患者,淋巴细胞计数减少了,有9位(6%)患者。154名患者中有13名(8%)患有3-5级与治疗相关的不良事件,其中最常见的是3名(2%)患者的淋巴细胞计数减少和2名(1%)患者的贫血。14名(9%)患者有与治疗相关的严重不良事件,最常见的是发热(4 [3%]),高血压和胸腔积液(各2 [1%])。4名患者(3%)因与治疗相关的不良事件而中止治疗,其中2例(1%)死亡(1例归因于肺水肿,1例归因于胸腔积液和肺炎)。在15例复发性或难治性霍奇金淋巴瘤患者中,2例完全缓解,7例部分缓解。因此,有9名患者达到了客观缓解(60·0%; 95%CI 32·3-83·7)。在136例实体瘤和其他淋巴瘤患者中,8例有部分反应(2例分别患有肾上腺皮质癌和间皮瘤,1例分别患有恶性神经胶质瘤,上皮样肉瘤,淋巴上皮癌和恶性横纹肌瘤);有客观反应的患者比例为5·9%(95%CI 2·6-11·3)。解释Pembrolizumab的耐受性良好,在复发或难治性霍奇金淋巴瘤的小儿患者中显示出令人鼓舞的抗肿瘤活性,与成人患者的经验一致。Pembrolizumab在大多数儿科肿瘤类型中均具有较低的抗肿瘤活性,并且仅在少数几种罕见的PD-L1阳性肿瘤类型中观察到了应答,这表明仅PD-L1表达不足以作为选择以下患者的儿科患者的生物标志物可能对PD-1检查点抑制剂有反应。KEYNOTE-051的最终结果预计将于2022年9月发布,并且有可能扩展,并将进一步报告派姆单抗在霍奇金淋巴瘤,微卫星不稳定性高肿瘤和黑色素瘤中的活性。资金默沙东(Merck Sharp&Dohme)是默克(Merck&Co.)的子公司。
更新日期:2020-01-04
中文翻译:

Pembrolizumab在患有晚期黑素瘤或PD-L1阳性,晚期,复发或难治性实体瘤或淋巴瘤的小儿患者中(KEYNOTE-051):一项开放性单臂1-2期试验的中期分析。
背景技术派姆单抗被批准用于治疗成人晚期癌症。但是,尚无有关儿科患者安全性和有效性的信息。我们的目标是确定推荐的2期剂量的派姆单抗及其在晚期小儿癌症中的安全性和抗肿瘤活性。方法KEYNOTE-051是一项正在进行的1-2期开放标签试验。在此中期分析中,从位于澳大利亚,巴西,加拿大,法国,德国,以色列,意大利,韩国,瑞典,英国和美国的30家医院中招募了6个月至17岁的儿童。黑色素瘤或中心确认的PD-L1阳性,复发或难治性实体瘤或淋巴瘤且Lansky Play / Karnofsky Performance状态评分为50或更高的患者,每3剂接受静脉注射pembrolizumab,初始剂量为2 mg / kg周。使用药代动力学和剂量限制性毒性确定推荐的2期剂量,并评估该剂量的安全性和抗肿瘤活性。主要终点是根据实体肿瘤1.1版或国际神经母细胞瘤反应标准中的反应评价标准,确定最大给药剂量,安全性和耐受性下的剂量限制性毒性以及每种肿瘤对pembrolizumab客观反应的患者比例。在接受至少一剂派姆单抗治疗的所有接受治疗的患者中评估安全性和有效性。复发或难治性经典霍奇金淋巴瘤患者队列的单独报告是事后决定。此中期分析的数据截止日期为2018年9月3日。该试验仍在招募患者,并已在ClinicalTrials.gov上注册,编号为NCT02332668。在2015年3月23日至2018年9月3日之间筛查的863例患者中,有796例可评估PD-L1表达的肿瘤(278例[35%]为PD-L1阳性); 纳入155位合格患者,其中154位患者接受至少一剂派姆单抗治疗。入组患者的中位年龄为13岁(IQR 8-15)。中位随访时间为8·6个月(IQR 2·5-16·4)。在第1阶段没有报道剂量限制性毒性,并且派姆单抗的血浆浓度与以前在成人中报道的浓度一致;因此,建议的第2阶段推荐剂量为每3周2 mg / kg。在接受治疗的154位患者中,有69位(占45%)经历了3-5级不良事件,最常见的是贫血,有14位(9%)患者,淋巴细胞计数减少了,有9位(6%)患者。154名患者中有13名(8%)患有3-5级与治疗相关的不良事件,其中最常见的是3名(2%)患者的淋巴细胞计数减少和2名(1%)患者的贫血。14名(9%)患者有与治疗相关的严重不良事件,最常见的是发热(4 [3%]),高血压和胸腔积液(各2 [1%])。4名患者(3%)因与治疗相关的不良事件而中止治疗,其中2例(1%)死亡(1例归因于肺水肿,1例归因于胸腔积液和肺炎)。在15例复发性或难治性霍奇金淋巴瘤患者中,2例完全缓解,7例部分缓解。因此,有9名患者达到了客观缓解(60·0%; 95%CI 32·3-83·7)。在136例实体瘤和其他淋巴瘤患者中,8例有部分反应(2例分别患有肾上腺皮质癌和间皮瘤,1例分别患有恶性神经胶质瘤,上皮样肉瘤,淋巴上皮癌和恶性横纹肌瘤);有客观反应的患者比例为5·9%(95%CI 2·6-11·3)。解释Pembrolizumab的耐受性良好,在复发或难治性霍奇金淋巴瘤的小儿患者中显示出令人鼓舞的抗肿瘤活性,与成人患者的经验一致。Pembrolizumab在大多数儿科肿瘤类型中均具有较低的抗肿瘤活性,并且仅在少数几种罕见的PD-L1阳性肿瘤类型中观察到了应答,这表明仅PD-L1表达不足以作为选择以下患者的儿科患者的生物标志物可能对PD-1检查点抑制剂有反应。KEYNOTE-051的最终结果预计将于2022年9月发布,并且有可能扩展,并将进一步报告派姆单抗在霍奇金淋巴瘤,微卫星不稳定性高肿瘤和黑色素瘤中的活性。资金默沙东(Merck Sharp&Dohme)是默克(Merck&Co.)的子公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号