当前位置:
X-MOL 学术
›
BBA Gen. Subj.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand binding and activation of UTP-activated G protein-coupled P2Y2 and P2Y4 receptors elucidated by mutagenesis, pharmacological and computational studies.
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.bbagen.2019.129501 Isaac Y Attah 1 , Alexander Neumann 1 , Haneen Al-Hroub 1 , Muhammad Rafehi 1 , Younis Baqi 2 , Vigneshwaran Namasivayam 1 , Christa E Müller 1
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.bbagen.2019.129501 Isaac Y Attah 1 , Alexander Neumann 1 , Haneen Al-Hroub 1 , Muhammad Rafehi 1 , Younis Baqi 2 , Vigneshwaran Namasivayam 1 , Christa E Müller 1
Affiliation
|
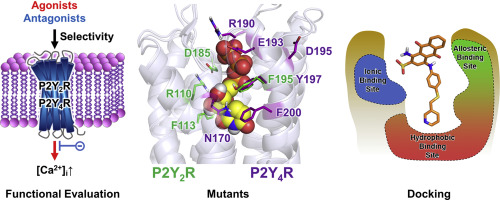
The nucleotide receptors P2Y2 and P2Y4 are the most closely related G protein-coupled receptors (GPCRs) of the P2Y receptor (P2YR) family. Both subtypes couple to Gq proteins and are activated by the pyrimidine nucleotide UTP, but only P2Y2R is also activated by the purine nucleotide ATP. Agonists and antagonists of both receptor subtypes have potential as drugs e.g. for neurodegenerative and inflammatory diseases. So far, potent and selective, "drug-like" ligands for both receptors are scarce, but would be required for target validation and as lead structures for drug development. Structural information on the receptors is lacking since no X-ray structures or cryo-electron microscopy images are available. Thus, we performed receptor homology modeling and docking studies combined with mutagenesis experiments on both receptors to address the question how ligand binding selectivity for these closely related P2YR subtypes can be achieved. The orthosteric binding site of P2Y2R appeared to be more spacious than that of P2Y4R. Mutation of Y197 to alanine in P2Y4R resulted in a gain of ATP sensitivity. Anthraquinone-derived antagonists are likely to bind to the orthosteric or an allosteric site depending on their substitution pattern and the nature of the orthosteric binding site of the respective P2YR subtype. These insights into the architecture of P2Y2- and P2Y4Rs and their interactions with structurally diverse agonists and antagonist provide a solid basis for the future design of potent and selective ligands.
中文翻译:

通过诱变,药理和计算研究阐明了UTP激活的G蛋白偶联的P2Y2和P2Y4受体的配体结合和激活。
核苷酸受体P2Y2和P2Y4是P2Y受体(P2YR)家族中关系最密切的G蛋白偶联受体(GPCR)。两种亚型均偶联至Gq蛋白,并被嘧啶核苷酸UTP激活,但只有P2Y2R也被嘌呤核苷酸ATP激活。两种受体亚型的激动剂和拮抗剂具有作为药物的潜力,例如用于神经退行性疾病和炎性疾病。到目前为止,两种受体的有效和选择性“药物样”配体都很稀缺,但需要用于靶标验证和药物开发的先导结构。缺乏关于受体的结构信息,因为没有X射线结构或低温电子显微镜图像。因此,我们对两种受体进行了受体同源性建模和对接研究,并进行了诱变实验,以解决如何实现对这些紧密相关的P2YR亚型的配体结合选择性的问题。P2Y2R的正构结合位点似乎比P2Y4R的更宽。P2Y4R中的Y197突变为丙氨酸导致ATP敏感性增加。蒽醌衍生的拮抗剂可能会结合到正构或变构位点,这取决于它们的取代方式和各个P2YR亚型的正构结合位点的性质。这些对P2Y2-和P2Y4Rs的结构及其与结构多样的激动剂和拮抗剂相互作用的见解为将来设计有效和选择性的配体提供了坚实的基础。
更新日期:2019-12-05
中文翻译:

通过诱变,药理和计算研究阐明了UTP激活的G蛋白偶联的P2Y2和P2Y4受体的配体结合和激活。
核苷酸受体P2Y2和P2Y4是P2Y受体(P2YR)家族中关系最密切的G蛋白偶联受体(GPCR)。两种亚型均偶联至Gq蛋白,并被嘧啶核苷酸UTP激活,但只有P2Y2R也被嘌呤核苷酸ATP激活。两种受体亚型的激动剂和拮抗剂具有作为药物的潜力,例如用于神经退行性疾病和炎性疾病。到目前为止,两种受体的有效和选择性“药物样”配体都很稀缺,但需要用于靶标验证和药物开发的先导结构。缺乏关于受体的结构信息,因为没有X射线结构或低温电子显微镜图像。因此,我们对两种受体进行了受体同源性建模和对接研究,并进行了诱变实验,以解决如何实现对这些紧密相关的P2YR亚型的配体结合选择性的问题。P2Y2R的正构结合位点似乎比P2Y4R的更宽。P2Y4R中的Y197突变为丙氨酸导致ATP敏感性增加。蒽醌衍生的拮抗剂可能会结合到正构或变构位点,这取决于它们的取代方式和各个P2YR亚型的正构结合位点的性质。这些对P2Y2-和P2Y4Rs的结构及其与结构多样的激动剂和拮抗剂相互作用的见解为将来设计有效和选择性的配体提供了坚实的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号