当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-Domain Antibodies as Crystallization Chaperones to Enable Structure-Based Inhibitor Development for RBR E3 Ubiquitin Ligases.
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.chembiol.2019.11.007 Yi-Chun Isabella Tsai 1 , Henrik Johansson 2 , David Dixon 3 , Stephen Martin 4 , Chun-Wa Chung 5 , Jane Clarkson 3 , David House 6 , Katrin Rittinger 1
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.chembiol.2019.11.007 Yi-Chun Isabella Tsai 1 , Henrik Johansson 2 , David Dixon 3 , Stephen Martin 4 , Chun-Wa Chung 5 , Jane Clarkson 3 , David House 6 , Katrin Rittinger 1
Affiliation
|
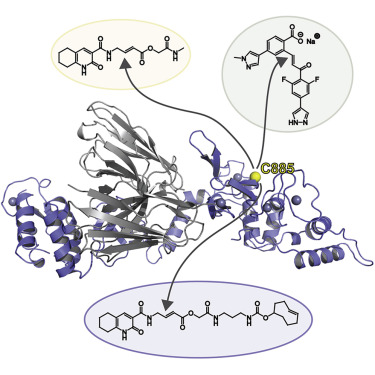
Protein ubiquitination plays a key role in the regulation of cellular processes, and misregulation of the ubiquitin system is linked to many diseases. So far, development of tool compounds that target enzymes of the ubiquitin system has been slow and only a few specific inhibitors are available. Here, we report the selection of single-domain antibodies (single-dAbs) based on a human scaffold that recognize the catalytic domain of HOIP, a subunit of the multi-component E3 LUBAC and member of the RBR family of E3 ligases. Some of these dAbs affect ligase activity and provide mechanistic insight into the ubiquitin transfer mechanism of different E2-conjugating enzymes. Furthermore, we show that the co-crystal structure of a HOIP RBR/dAb complex serves as a robust platform for soaking of ligands that target the active site cysteine of HOIP, thereby providing easy access to structure-based ligand design for this important class of E3 ligases.
中文翻译:

单域抗体作为结晶伴侣,可实现 RBR E3 泛素连接的基于结构的抑制剂开发。
蛋白质泛素化在细胞过程的调节中起着关键作用,泛素系统的失调与许多疾病有关。到目前为止,针对泛素系统酶的工具化合物的开发进展缓慢,并且只有少数特定的抑制剂可用。在这里,我们报告了基于人类支架的单域抗体 (single-dAb) 的选择,该抗体识别 HOIP 的催化域,HOIP 是多组分 E3 LUBAC 的亚基,也是 E3 连接酶 RBR 家族的成员。其中一些 dAb 会影响连接酶活性,并提供对不同 E2 结合酶的泛素转移机制的深入了解。此外,我们还表明,HOIP RBR/dAb 复合物的共晶结构可作为浸泡靶向 HOIP 活性位点半胱氨酸的配体的强大平台,从而为这一类重要的药物提供基于结构的配体设计。 E3 连接酶。
更新日期:2019-12-05
中文翻译:

单域抗体作为结晶伴侣,可实现 RBR E3 泛素连接的基于结构的抑制剂开发。
蛋白质泛素化在细胞过程的调节中起着关键作用,泛素系统的失调与许多疾病有关。到目前为止,针对泛素系统酶的工具化合物的开发进展缓慢,并且只有少数特定的抑制剂可用。在这里,我们报告了基于人类支架的单域抗体 (single-dAb) 的选择,该抗体识别 HOIP 的催化域,HOIP 是多组分 E3 LUBAC 的亚基,也是 E3 连接酶 RBR 家族的成员。其中一些 dAb 会影响连接酶活性,并提供对不同 E2 结合酶的泛素转移机制的深入了解。此外,我们还表明,HOIP RBR/dAb 复合物的共晶结构可作为浸泡靶向 HOIP 活性位点半胱氨酸的配体的强大平台,从而为这一类重要的药物提供基于结构的配体设计。 E3 连接酶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号