Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS.
Cell ( IF 45.5 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.cell.2019.11.016 Chun-Cheih Chao 1 , Cristina Gutiérrez-Vázquez 1 , Veit Rothhammer 2 , Lior Mayo 3 , Michael A Wheeler 1 , Emily C Tjon 1 , Stephanie E J Zandee 4 , Manon Blain 5 , Kalil Alves de Lima 1 , Maisa C Takenaka 1 , Julian Avila-Pacheco 6 , Patrick Hewson 1 , Lei Liu 1 , Liliana M Sanmarco 1 , Davis M Borucki 1 , Gabriel Z Lipof 1 , Sunia A Trauger 7 , Clary B Clish 6 , Jack P Antel 5 , Alexandre Prat 8 , Francisco J Quintana 9
Cell ( IF 45.5 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.cell.2019.11.016 Chun-Cheih Chao 1 , Cristina Gutiérrez-Vázquez 1 , Veit Rothhammer 2 , Lior Mayo 3 , Michael A Wheeler 1 , Emily C Tjon 1 , Stephanie E J Zandee 4 , Manon Blain 5 , Kalil Alves de Lima 1 , Maisa C Takenaka 1 , Julian Avila-Pacheco 6 , Patrick Hewson 1 , Lei Liu 1 , Liliana M Sanmarco 1 , Davis M Borucki 1 , Gabriel Z Lipof 1 , Sunia A Trauger 7 , Clary B Clish 6 , Jack P Antel 5 , Alexandre Prat 8 , Francisco J Quintana 9
Affiliation
|
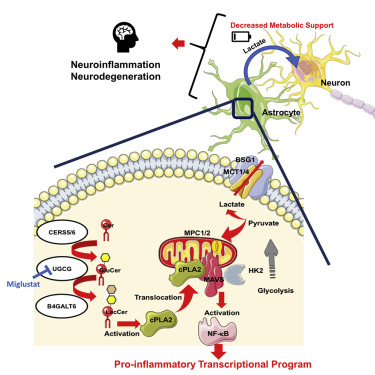
Metabolism has been shown to control peripheral immunity, but little is known about its role in central nervous system (CNS) inflammation. Through a combination of proteomic, metabolomic, transcriptomic, and perturbation studies, we found that sphingolipid metabolism in astrocytes triggers the interaction of the C2 domain in cytosolic phospholipase A2 (cPLA2) with the CARD domain in mitochondrial antiviral signaling protein (MAVS), boosting NF-κB-driven transcriptional programs that promote CNS inflammation in experimental autoimmune encephalomyelitis (EAE) and, potentially, multiple sclerosis. cPLA2 recruitment to MAVS also disrupts MAVS-hexokinase 2 (HK2) interactions, decreasing HK enzymatic activity and the production of lactate involved in the metabolic support of neurons. Miglustat, a drug used to treat Gaucher and Niemann-Pick disease, suppresses astrocyte pathogenic activities and ameliorates EAE. Collectively, these findings define a novel immunometabolic mechanism that drives pro-inflammatory astrocyte activities, outlines a new role for MAVS in CNS inflammation, and identifies candidate targets for therapeutic intervention.
中文翻译:

通过cPLA2-MAVS的星形胶质细胞致病活性的代谢控制。
代谢已显示出控制外周免疫的作用,但对其在中枢神经系统(CNS)炎症中的作用知之甚少。通过蛋白质组学,代谢组学,转录组学和摄动研究的组合,我们发现星形胶质细胞中的鞘脂代谢触发线粒体抗病毒信号蛋白(MAVS)中胞质磷脂酶A2(cPLA2)的C2结构域与CARD结构域的相互作用。 -κB驱动的转录程序,可在实验性自身免疫性脑脊髓炎(EAE)以及潜在的多发性硬化症中促进中枢神经系统炎症。cPLA2募集到MAVS还破坏了MAVS-己糖激酶2(HK2)的相互作用,降低了HK的酶活性和涉及神经元代谢支持的乳酸的产生。Miglustat,一种用于治疗Gaucher和Niemann-Pick疾病的药物,抑制星形胶质细胞的致病活性并改善EAE。总的来说,这些发现定义了一种新的免疫代谢机制,可驱动促炎性星形胶质细胞活动,概述MAVS在中枢神经系统炎症中的新作用,并确定治疗干预的候选靶点。
更新日期:2019-12-05
中文翻译:

通过cPLA2-MAVS的星形胶质细胞致病活性的代谢控制。
代谢已显示出控制外周免疫的作用,但对其在中枢神经系统(CNS)炎症中的作用知之甚少。通过蛋白质组学,代谢组学,转录组学和摄动研究的组合,我们发现星形胶质细胞中的鞘脂代谢触发线粒体抗病毒信号蛋白(MAVS)中胞质磷脂酶A2(cPLA2)的C2结构域与CARD结构域的相互作用。 -κB驱动的转录程序,可在实验性自身免疫性脑脊髓炎(EAE)以及潜在的多发性硬化症中促进中枢神经系统炎症。cPLA2募集到MAVS还破坏了MAVS-己糖激酶2(HK2)的相互作用,降低了HK的酶活性和涉及神经元代谢支持的乳酸的产生。Miglustat,一种用于治疗Gaucher和Niemann-Pick疾病的药物,抑制星形胶质细胞的致病活性并改善EAE。总的来说,这些发现定义了一种新的免疫代谢机制,可驱动促炎性星形胶质细胞活动,概述MAVS在中枢神经系统炎症中的新作用,并确定治疗干预的候选靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号