Tetrahedron ( IF 2.1 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.tet.2019.130842 Sandra Hernández-Ibáñez , Olga Soares do Rego Barros , Alejandro Lahosa , María Jesús García-Muñoz , Meriem Benlahrech , Cherif Behloul , Francisco Foubelo , Miguel Yus
|
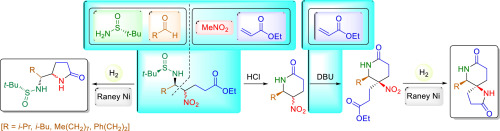
The reaction of N-tert-butanesulfinyl imines with ethyl 4-nitrobutanoate under basic conditions produced nitro amine derivatives. The resulting β-nitroamine derivatives, isolated as a 1:1 mixture of epimers, were easily transformed into 5-(1-aminoalkyl)-2-pyrrolidones, upon reduction of the nitro group with concomitant γ-lactam formation. On the other hand, selective removal of the sulfinyl group in the β-nitroamine derivatives led to 5-nitropiperidin-2-ones in reasonable yields. From these compounds, and following a two-step process, involving conjugative addition to ethyl acrylate and final reduction of the nitro group, 1,7-diazaspiro[4.5]decane-2,8-diones were accessed in a highly stereoselective fashion.
中文翻译:

由手性N-叔-丁烷亚磺酰基亚胺和4-硝基丁酸乙酯立体选择性合成5-(1-氨基烷基)-2-吡咯烷酮和1,7-二氮杂螺[4.5]癸烷-2,8-二酮
的反应ñ -叔产生硝基胺衍生物在碱性条件下-butanesulfinyl亚胺与4- nitrobutanoate。分离为1:1差向异构体混合物的所得β-硝基胺衍生物,在硝基还原并伴有γ-内酰胺形成的情况下,很容易转化为5-(1-氨基烷基)-2-吡咯烷酮。另一方面,选择性除去β-硝基胺衍生物中的亚磺酰基导致以合理的收率得到5-硝基哌啶-2-酮。从这些化合物开始,并经过两步过程,包括向丙烯酸乙酯中的共轭加成和硝基的最终还原,以高度立体选择性的方式获得了1,7-二氮杂螺并[4.5]癸烷-2,8-二酮。



























 京公网安备 11010802027423号
京公网安备 11010802027423号