当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Intrinsically Disordered Regions in Acceleration of Protein-Protein Association.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jpcb.9b08793 Mikita M. Misiura , Anatoly B. Kolomeisky
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jpcb.9b08793 Mikita M. Misiura , Anatoly B. Kolomeisky
|
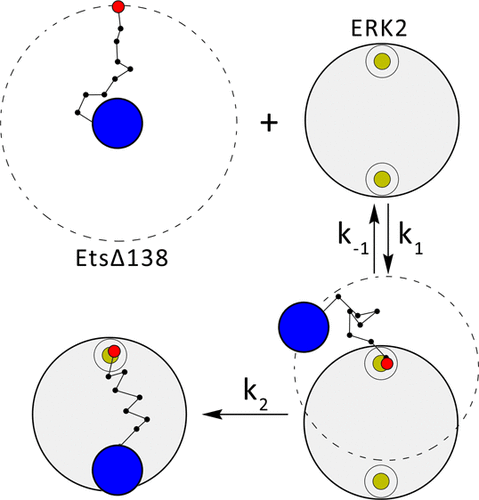
Although intrinsically disordered proteins and intrinsically disordered regions (IDRs) in folded proteins are not able to form stable structures, it is known that they play critically important roles in various biological processes. However, despite multiple studies, the molecular mechanisms of their functions remain not fully understood. In this work, we theoretically investigate the role of IDRs in acceleration of protein-protein association processes. Our hypothesis is that, in protein pairs with several independent binding sites, the association process goes faster if some of these binding sites are located on IDRs or connected by IDRs. To test this idea, we employed analytical modeling, numerical calculations, and Brownian dynamics computer simulations to calculate protein-protein association reaction rates for the ERK2-EtsΔ138 system, belonging to the RAS-RAF-MEK-ERK signaling pathway in living cells. It is found that putting a binding site on IDR accelerates the association process by a factor of 3 to 4. Possible molecular explanations for these observations are presented, and other systems that might use this mechanism are also mentioned.
中文翻译:

内在失调区域在促进蛋白质-蛋白质结合中的作用。
尽管折叠蛋白中的固有无序蛋白和固有无序区(IDR)无法形成稳定的结构,但众所周知它们在各种生物学过程中都起着至关重要的作用。然而,尽管进行了多次研究,但对其功能的分子机制仍未完全了解。在这项工作中,我们从理论上研究了IDR在加速蛋白质-蛋白质缔合过程中的作用。我们的假设是,在具有几个独立结合位点的蛋白质对中,如果其中一些结合位点位于IDR上或通过IDR连接,则缔合过程会更快。为了验证这一想法,我们采用了分析模型,数值计算和布朗动力学计算机仿真来计算ERK2-EtsΔ138系统的蛋白质-蛋白质缔合反应速率,属于活细胞中的RAS-RAF-MEK-ERK信号通路。发现在IDR上放置一个结合位点可以使结合过程加快3到4倍。给出了这些观察结果的可能分子解释,并且还提到了其他可能使用此机制的系统。
更新日期:2019-12-19
中文翻译:

内在失调区域在促进蛋白质-蛋白质结合中的作用。
尽管折叠蛋白中的固有无序蛋白和固有无序区(IDR)无法形成稳定的结构,但众所周知它们在各种生物学过程中都起着至关重要的作用。然而,尽管进行了多次研究,但对其功能的分子机制仍未完全了解。在这项工作中,我们从理论上研究了IDR在加速蛋白质-蛋白质缔合过程中的作用。我们的假设是,在具有几个独立结合位点的蛋白质对中,如果其中一些结合位点位于IDR上或通过IDR连接,则缔合过程会更快。为了验证这一想法,我们采用了分析模型,数值计算和布朗动力学计算机仿真来计算ERK2-EtsΔ138系统的蛋白质-蛋白质缔合反应速率,属于活细胞中的RAS-RAF-MEK-ERK信号通路。发现在IDR上放置一个结合位点可以使结合过程加快3到4倍。给出了这些观察结果的可能分子解释,并且还提到了其他可能使用此机制的系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号