当前位置:
X-MOL 学术
›
Gastroenterology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension.
Gastroenterology ( IF 25.7 ) Pub Date : 2019-12-05 , DOI: 10.1053/j.gastro.2019.11.296 Naga Chalasani 1 , Manal F Abdelmalek 2 , Guadalupe Garcia-Tsao 3 , Raj Vuppalanchi 1 , Naim Alkhouri 4 , Mary Rinella 5 , Mazen Noureddin 6 , Maxmillan Pyko 1 , Mitchell Shiffman 7 , Arun Sanyal 8 , Adam Allgood 9 , Harold Shlevin 9 , Rex Horton 9 , Eliezer Zomer 9 , William Irish 10 , Zachary Goodman 11 , Stephen A Harrison 12 , Peter G Traber 9 ,
Gastroenterology ( IF 25.7 ) Pub Date : 2019-12-05 , DOI: 10.1053/j.gastro.2019.11.296 Naga Chalasani 1 , Manal F Abdelmalek 2 , Guadalupe Garcia-Tsao 3 , Raj Vuppalanchi 1 , Naim Alkhouri 4 , Mary Rinella 5 , Mazen Noureddin 6 , Maxmillan Pyko 1 , Mitchell Shiffman 7 , Arun Sanyal 8 , Adam Allgood 9 , Harold Shlevin 9 , Rex Horton 9 , Eliezer Zomer 9 , William Irish 10 , Zachary Goodman 11 , Stephen A Harrison 12 , Peter G Traber 9 ,
Affiliation
|
BACKGROUND & AIMS
Increased levels of galectin 3 have been associated with nonalcoholic steatohepatitis (NASH) and contribute to toxin-induced liver fibrosis in mice. GR-MD-02 (belapectin) is an inhibitor of galectin 3 that reduces liver fibrosis and portal hypertension in rats and was safe and well tolerated in phase 1 studies. We performed a phase 2b, randomized trial of the safety and efficacy of GR-MD-02 in patients with NASH, cirrhosis, and portal hypertension.
METHODS
Patients with NASH, cirrhosis, and portal hypertension (hepatic venous pressure gradient [HVPG] ≥ 6 mm Hg) from 36 centers were randomly assigned, in a double-blind manner, to groups that received biweekly infusions of belapectin 2 mg/kg (n = 54), 8 mg/kg (n = 54), or placebo (n = 54) for 52 weeks. The primary endpoint was change in HVPG (-28) at the end of the 52-week period compared with baseline. Secondary endpoints included changes in liver histology and development of liver-related outcomes.
RESULTS
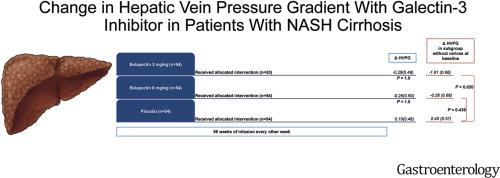
We found no significant difference in ΔHVPG between the 2 mg/kg belapectin group and placebo group (-0.28 mm HG vs 0.10 mm HG, P = 1.0) or between the 8 mg/kg belapectin and placebo group (-0.25 mm HG vs 0.10 mm HG, P = 1.0). Belapectin had no significant effect on fibrosis or nonalcoholic fatty liver disease activity score, and liver-related outcomes did not differ significantly among groups. In an analysis of a subgroup of patients without esophageal varices at baseline (n = 81), 2 mg/kg belapectin was associated with a reduction in HVPG at 52 weeks compared with baseline (P = .02) and reduced development of new varices (P = .03). Belapectin (2 mg/kg) was well tolerated and produced no safety signals.
CONCLUSIONS
In a phase 2b study of 162 patients with NASH, cirrhosis, and portal hypertension, 1 year of biweekly infusion of belapectin was safe but not associated with significant reduction in HVPG or fibrosis compared with placebo. However, in a subgroup analysis of patients without esophageal varices, 2 mg/kg belapectin did reduce HVPG and development of varices. ClinicalTrials.gov number: NCT02462967.
中文翻译:

Belapectin(一种Galectin-3抑制剂)对非酒精性脂肪性肝炎合并肝硬化和门脉高压的疗效。
背景与目的galectin 3的水平升高与非酒精性脂肪性肝炎(NASH)有关,并导致毒素诱导的小鼠肝纤维化。GR-MD-02(belapectin)是半乳糖凝集素3(galectin 3)的抑制剂,可减轻大鼠的肝纤维化和门脉高压,在1期研究中安全且耐受性良好。我们进行了2b期GR-MD-02在NASH,肝硬化和门静脉高压症患者中的安全性和有效性的随机试验。方法将来自36个中心的NASH,肝硬化和门静脉高压症(肝静脉压力梯度[HVPG]≥6 mm Hg]的患者以双盲方式随机分配到每两周一次接受belapectin 2 mg / kg输注的组( n = 54),8 mg / kg(n = 54)或安慰剂(n = 54)持续52周。主要终点是与基线相比在52周结束时HVPG(-28)的变化。次要终点包括肝脏组织学变化和肝脏相关结局的发展。结果我们发现2 mg / kg贝拉胶蛋白组和安慰剂组之间的ΔHVPG差异无显着性(-0.28 mm HG vs.0.10 mm HG,P = 1.0)或8 mg / kg贝拉胶蛋白和安慰剂组(-0.25 mm HGvs。 0.10 mm HG,P = 1.0)。Belapectin对纤维化或非酒精性脂肪肝疾病活动度评分无显着影响,并且各组之间与肝脏相关的结局无显着差异。在基线时无食管静脉曲张的亚组患者中进行分析(n = 81),与基线相比,52周时2 mg / kg贝拉克汀与HVPG降低有关(P = .02),新静脉曲张的发生减少( P = .03)。Belapectin(2 mg / kg)的耐受性良好,未产生安全信号。结论在一项针对162例NASH,肝硬化和门静脉高压症患者的2b期研究中,与安慰剂相比,每两周输注贝拉果胶1年是安全的,但与HVPG或纤维化的明显降低无关。但是,在对没有食管静脉曲张的患者进行的亚组分析中,2 mg / kg贝拉克汀确实降低了HVPG和静脉曲张的发生。ClinicalTrials.gov编号:NCT02462967。2 mg / kg的belapectin确实降低了HVPG和静脉曲张的发展。ClinicalTrials.gov编号:NCT02462967。2 mg / kg的belapectin确实降低了HVPG和静脉曲张的发展。ClinicalTrials.gov编号:NCT02462967。
更新日期:2020-04-21
中文翻译:

Belapectin(一种Galectin-3抑制剂)对非酒精性脂肪性肝炎合并肝硬化和门脉高压的疗效。
背景与目的galectin 3的水平升高与非酒精性脂肪性肝炎(NASH)有关,并导致毒素诱导的小鼠肝纤维化。GR-MD-02(belapectin)是半乳糖凝集素3(galectin 3)的抑制剂,可减轻大鼠的肝纤维化和门脉高压,在1期研究中安全且耐受性良好。我们进行了2b期GR-MD-02在NASH,肝硬化和门静脉高压症患者中的安全性和有效性的随机试验。方法将来自36个中心的NASH,肝硬化和门静脉高压症(肝静脉压力梯度[HVPG]≥6 mm Hg]的患者以双盲方式随机分配到每两周一次接受belapectin 2 mg / kg输注的组( n = 54),8 mg / kg(n = 54)或安慰剂(n = 54)持续52周。主要终点是与基线相比在52周结束时HVPG(-28)的变化。次要终点包括肝脏组织学变化和肝脏相关结局的发展。结果我们发现2 mg / kg贝拉胶蛋白组和安慰剂组之间的ΔHVPG差异无显着性(-0.28 mm HG vs.0.10 mm HG,P = 1.0)或8 mg / kg贝拉胶蛋白和安慰剂组(-0.25 mm HGvs。 0.10 mm HG,P = 1.0)。Belapectin对纤维化或非酒精性脂肪肝疾病活动度评分无显着影响,并且各组之间与肝脏相关的结局无显着差异。在基线时无食管静脉曲张的亚组患者中进行分析(n = 81),与基线相比,52周时2 mg / kg贝拉克汀与HVPG降低有关(P = .02),新静脉曲张的发生减少( P = .03)。Belapectin(2 mg / kg)的耐受性良好,未产生安全信号。结论在一项针对162例NASH,肝硬化和门静脉高压症患者的2b期研究中,与安慰剂相比,每两周输注贝拉果胶1年是安全的,但与HVPG或纤维化的明显降低无关。但是,在对没有食管静脉曲张的患者进行的亚组分析中,2 mg / kg贝拉克汀确实降低了HVPG和静脉曲张的发生。ClinicalTrials.gov编号:NCT02462967。2 mg / kg的belapectin确实降低了HVPG和静脉曲张的发展。ClinicalTrials.gov编号:NCT02462967。2 mg / kg的belapectin确实降低了HVPG和静脉曲张的发展。ClinicalTrials.gov编号:NCT02462967。











































 京公网安备 11010802027423号
京公网安备 11010802027423号