当前位置:
X-MOL 学术
›
Toxicol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of the fentanyl analog screening kit toward the identification of emerging synthetic opioids in human plasma and urine by LC-QTOF
Toxicology Letters ( IF 2.9 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.toxlet.2019.12.007 Logan C Krajewski 1 , Kenneth D Swanson 2 , William A Bragg 2 , Rebecca L Shaner 2 , Craig Seymour 2 , Melissa D Carter 2 , Elizabeth I Hamelin 2 , Rudolph C Johnson 2
Toxicology Letters ( IF 2.9 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.toxlet.2019.12.007 Logan C Krajewski 1 , Kenneth D Swanson 2 , William A Bragg 2 , Rebecca L Shaner 2 , Craig Seymour 2 , Melissa D Carter 2 , Elizabeth I Hamelin 2 , Rudolph C Johnson 2
Affiliation
|
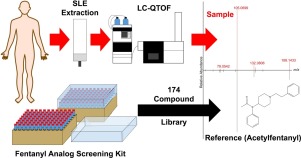
Human exposures to fentanyl analogs, which significantly contribute to the ongoing U.S. opioid overdose epidemic, can be confirmed through the analysis of clinical samples. Our laboratory has developed and evaluated a qualitative approach coupling liquid chromatography and quadrupole time-of-flight mass spectrometry (LC-QTOF) to address novel fentanyl analogs and related compounds using untargeted, data-dependent acquisition. Compound identification was accomplished by searching against a locally-established mass spectral library of 174 fentanyl analogs and metabolites. Currently, our library can identify 150 fentanyl-related compounds from the Fentanyl Analog Screening (FAS) Kit), plus an additional 25 fentanyl-related compounds from individual purchases. Plasma and urine samples fortified with fentanyl-related compounds were assessed to confirm the capabilities and intended use of this LC-QTOF method. For fentanyl, 8 fentanyl-related compounds and naloxone, lower reportable limits (LRL100), defined as the lowest concentration with 100 % true positive rate (n = 12) within clinical samples, were evaluated and range from 0.5 ng/mL to 5.0 ng/mL for urine and 0.25 ng/mL to 2.5 ng/mL in plasma. The application of this high resolution mass spectrometry (HRMS) method enables the real-time detection of known and emerging synthetic opioids present in clinical samples.
中文翻译:

芬太尼类似物筛选试剂盒在 LC-QTOF 鉴定人血浆和尿液中新兴合成阿片类药物中的应用
人类暴露于芬太尼类似物,这对美国持续的阿片类药物过量流行有重大贡献,可以通过临床样本分析来确认。我们的实验室开发并评估了一种定性方法,将液相色谱与四极杆飞行时间质谱 (LC-QTOF) 结合使用,以使用非靶向、数据相关的采集来处理新型芬太尼类似物和相关化合物。化合物鉴定是通过搜索本地建立的 174 种芬太尼类似物和代谢物的质谱库来完成的。目前,我们的库可以从芬太尼类似物筛选 (FAS) 试剂盒中识别 150 种芬太尼相关化合物,另外还有 25 种来自个人购买的芬太尼相关化合物。对添加了芬太尼相关化合物的血浆和尿液样本进行了评估,以确认该 LC-QTOF 方法的能力和预期用途。对于芬太尼,评估了 8 种芬太尼相关化合物和纳洛酮的可报告下限 (LRL100),定义为临床样品中 100% 真阳性率 (n = 12) 的最低浓度,范围为 0.5 ng/mL 至 5.0 ng /mL 尿液和 0.25 ng/mL 至 2.5 ng/mL 血浆。这种高分辨率质谱 (HRMS) 方法的应用能够实时检测临床样本中存在的已知和新兴合成阿片类药物。定义为临床样本中 100% 真阳性率 (n = 12) 的最低浓度,进行评估,尿液浓度范围为 0.5 ng/mL 至 5.0 ng/mL,血浆浓度范围为 0.25 ng/mL 至 2.5 ng/mL。这种高分辨率质谱 (HRMS) 方法的应用能够实时检测临床样本中存在的已知和新兴合成阿片类药物。定义为临床样本中 100% 真阳性率 (n = 12) 的最低浓度,进行评估,尿液浓度范围为 0.5 ng/mL 至 5.0 ng/mL,血浆浓度范围为 0.25 ng/mL 至 2.5 ng/mL。这种高分辨率质谱 (HRMS) 方法的应用能够实时检测临床样本中存在的已知和新兴合成阿片类药物。
更新日期:2020-03-01
中文翻译:

芬太尼类似物筛选试剂盒在 LC-QTOF 鉴定人血浆和尿液中新兴合成阿片类药物中的应用
人类暴露于芬太尼类似物,这对美国持续的阿片类药物过量流行有重大贡献,可以通过临床样本分析来确认。我们的实验室开发并评估了一种定性方法,将液相色谱与四极杆飞行时间质谱 (LC-QTOF) 结合使用,以使用非靶向、数据相关的采集来处理新型芬太尼类似物和相关化合物。化合物鉴定是通过搜索本地建立的 174 种芬太尼类似物和代谢物的质谱库来完成的。目前,我们的库可以从芬太尼类似物筛选 (FAS) 试剂盒中识别 150 种芬太尼相关化合物,另外还有 25 种来自个人购买的芬太尼相关化合物。对添加了芬太尼相关化合物的血浆和尿液样本进行了评估,以确认该 LC-QTOF 方法的能力和预期用途。对于芬太尼,评估了 8 种芬太尼相关化合物和纳洛酮的可报告下限 (LRL100),定义为临床样品中 100% 真阳性率 (n = 12) 的最低浓度,范围为 0.5 ng/mL 至 5.0 ng /mL 尿液和 0.25 ng/mL 至 2.5 ng/mL 血浆。这种高分辨率质谱 (HRMS) 方法的应用能够实时检测临床样本中存在的已知和新兴合成阿片类药物。定义为临床样本中 100% 真阳性率 (n = 12) 的最低浓度,进行评估,尿液浓度范围为 0.5 ng/mL 至 5.0 ng/mL,血浆浓度范围为 0.25 ng/mL 至 2.5 ng/mL。这种高分辨率质谱 (HRMS) 方法的应用能够实时检测临床样本中存在的已知和新兴合成阿片类药物。定义为临床样本中 100% 真阳性率 (n = 12) 的最低浓度,进行评估,尿液浓度范围为 0.5 ng/mL 至 5.0 ng/mL,血浆浓度范围为 0.25 ng/mL 至 2.5 ng/mL。这种高分辨率质谱 (HRMS) 方法的应用能够实时检测临床样本中存在的已知和新兴合成阿片类药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号