Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Convenient One-Pot Synthesis of Both Enantiomers of 1,3,5-Trisubstituted Hydantoins
Synlett ( IF 1.7 ) Pub Date : 2019-12-04 , DOI: 10.1055/s-0039-1691406 Gun Hee Han , Seo Yun Kim , Ha Rim Lee , Ji Su Lee , Yong Sun Park 1
Synlett ( IF 1.7 ) Pub Date : 2019-12-04 , DOI: 10.1055/s-0039-1691406 Gun Hee Han , Seo Yun Kim , Ha Rim Lee , Ji Su Lee , Yong Sun Park 1
Affiliation
|
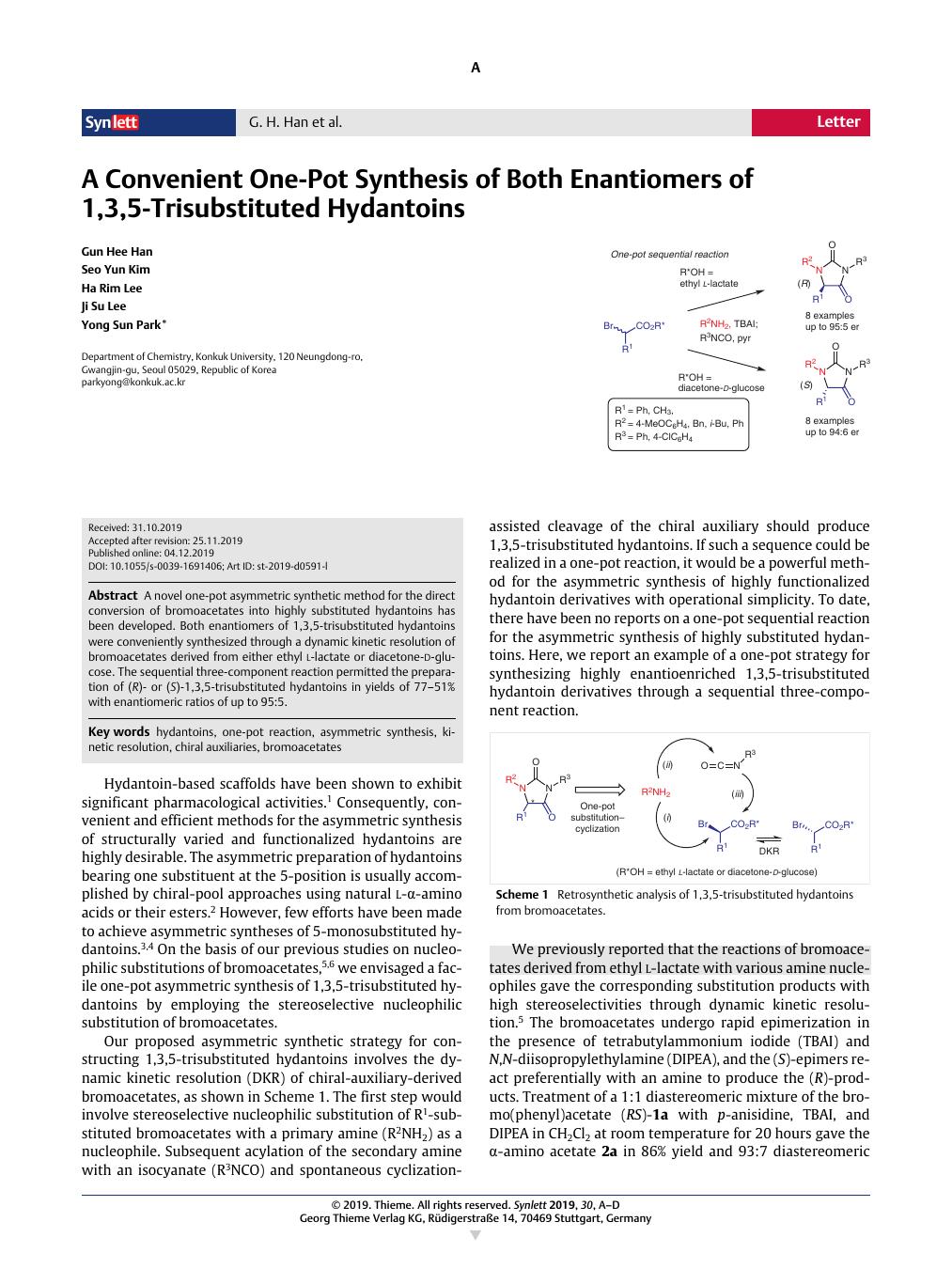
A novel one-pot asymmetric synthetic method for the direct conversion of bromoacetates into highly substituted hydantoins has been developed. Both enantiomers of 1,3,5-trisubstituted hydantoins were conveniently synthesized through a dynamic kinetic resolution of bromoacetates derived from either ethyl l -lactate or diacetone- d -glucose. The sequential three-component reaction permitted the preparation of (R)- or (S)-1,3,5-trisubstituted hydantoins in yields of 77–51% with enantiomeric ratios of up to 95:5.
中文翻译:

1,3,5-三取代乙内酰脲的两种对映异构体的方便的一锅法合成
开发了一种将溴乙酸盐直接转化为高度取代的乙内酰脲的新型单锅不对称合成方法。1,3,5-三取代乙内酰脲的两种对映异构体通过动态动力学拆分衍生自 l-乳酸乙酯或双丙酮-d-葡萄糖的溴乙酸盐方便地合成。连续三组分反应允许以 77-51% 的产率制备 (R)- 或 (S)-1,3,5- 三取代的乙内酰脲,对映体比例高达 95:5。
更新日期:2019-12-04
中文翻译:

1,3,5-三取代乙内酰脲的两种对映异构体的方便的一锅法合成
开发了一种将溴乙酸盐直接转化为高度取代的乙内酰脲的新型单锅不对称合成方法。1,3,5-三取代乙内酰脲的两种对映异构体通过动态动力学拆分衍生自 l-乳酸乙酯或双丙酮-d-葡萄糖的溴乙酸盐方便地合成。连续三组分反应允许以 77-51% 的产率制备 (R)- 或 (S)-1,3,5- 三取代的乙内酰脲,对映体比例高达 95:5。











































 京公网安备 11010802027423号
京公网安备 11010802027423号