当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
LRRC8/VRAC channels exhibit a noncanonical permeability to glutathione, which modulates epithelial-to-mesenchymal transition (EMT).
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-12-05 , DOI: 10.1038/s41419-019-2167-z Jonas Friard 1 , Alain Corinus 1 , Marc Cougnon 1 , Michel Tauc 1 , Didier F Pisani 1 , Christophe Duranton 1 , Isabelle Rubera 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-12-05 , DOI: 10.1038/s41419-019-2167-z Jonas Friard 1 , Alain Corinus 1 , Marc Cougnon 1 , Michel Tauc 1 , Didier F Pisani 1 , Christophe Duranton 1 , Isabelle Rubera 1
Affiliation
|
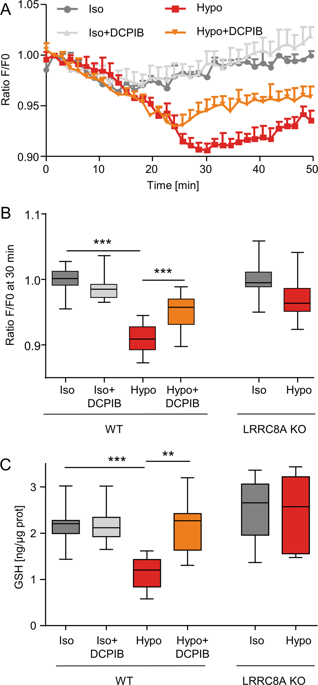
Volume-regulated anion channels (VRAC) are chloride channels activated in response to osmotic stress to regulate cellular volume and also participate in other cellular processes, including cell division and cell death. Recently, members of the LRRC8 family have been identified as the main contributors of VRAC conductance. LRRC8/VRAC is permeable to chloride ions but also exhibits significant permeability to various substrates that vary strongly in charge and size. In this study, we explored the intriguing ability of LRRC8/VRAC to transport glutathione (GSH), the major cellular reactive oxygen species (ROS) scavenger, and its involvement in epithelial-to-mesenchymal transition (EMT), a cellular process in which cellular oxidative status is a crucial step. First, in HEK293-WT cells, we showed that a hypotonic condition induced LRRC8/VRAC-dependent GSH conductance (PGSH/PCl of ~0.1) and a marked decrease in intracellular GSH content. GSH currents and GSH intracellular decrease were both inhibited by DCPIB, an inhibitor of LRRC8/VRAC, and were not observed in HEK293-LRRC8A KO cells. Then, we induced EMT by exposing renal proximal tubule epithelial cells to the pleiotropic growth factor TGFβ1, and we measured the contribution of LRRC8/VRAC in this process by measuring (i) EMT marker expression (assessed both at the gene and protein levels), (ii) cell morphology and (iii) the increase in migration ability. Interestingly, pharmacologic targeting of LRRC8/VRAC (DCPIB) or RNA interference-mediated inhibition (LRRC8A siRNA) attenuated the TGFβ1-induced EMT response by controlling GSH and ROS levels. Interestingly, TGFβ1 exposure triggered DCPIB-sensitive chloride conductance. These results suggest that LRRC8/VRAC, due to its native permeability to GSH and thus its ability to modulate ROS levels, plays a critical role in EMT and might contribute to other physiological and pathophysiological processes associated with oxidative stress.
中文翻译:

LRRC8 / VRAC通道对谷胱甘肽具有非规范的渗透性,可调节上皮-间充质转化(EMT)。
体积调节阴离子通道(VRAC)是响应渗透压而激活的氯离子通道,可调节细胞体积,并参与其他细胞过程,包括细胞分裂和细胞死亡。最近,已确定LRRC8家族的成员是VRAC电导的主要贡献者。LRRC8 / VRAC对氯离子具有渗透性,但对电荷和尺寸变化很大的各种底物也显示出显着的渗透性。在这项研究中,我们探索了LRRC8 / VRAC转运谷胱甘肽(GSH),主要的细胞活性氧(ROS)清除剂的有趣能力,以及它参与上皮到间质转化(EMT)的过程,其中细胞的氧化状态是至关重要的一步。首先,在HEK293-WT细胞中 我们表明低渗条件诱导LRRC8 / VRAC依赖性GSH电导(PGSH / PCl为〜0.1),并且细胞内GSH含量显着降低。GSH电流和GSH细胞内减少都被DCPIB(LRRC8 / VRAC的抑制剂)抑制,在HEK293-LRRC8A KO细胞中未观察到。然后,我们通过将肾小管上皮细胞暴露于多效性生长因子TGFβ1来诱导EMT,并通过测量(i)EMT标记物表达(在基因和蛋白质水平上进行评估)来测量LRRC8 / VRAC在此过程中的贡献, (ii)细胞形态和(iii)迁移能力的提高。有趣的是,药理靶向LRRC8 / VRAC(DCPIB)或RNA干扰介导的抑制(LRRC8A siRNA)通过控制GSH和ROS水平减弱了TGFβ1诱导的EMT反应。有趣的是,TGFβ1的暴露触发了DCPIB敏感的氯化物电导。这些结果表明,LRRC8 / VRAC由于其对GSH的天然渗透性,因而具有调节ROS水平的能力,因此在EMT中起关键作用,并可能有助于与氧化应激相关的其他生理和病理生理过程。
更新日期:2019-12-05
中文翻译:

LRRC8 / VRAC通道对谷胱甘肽具有非规范的渗透性,可调节上皮-间充质转化(EMT)。
体积调节阴离子通道(VRAC)是响应渗透压而激活的氯离子通道,可调节细胞体积,并参与其他细胞过程,包括细胞分裂和细胞死亡。最近,已确定LRRC8家族的成员是VRAC电导的主要贡献者。LRRC8 / VRAC对氯离子具有渗透性,但对电荷和尺寸变化很大的各种底物也显示出显着的渗透性。在这项研究中,我们探索了LRRC8 / VRAC转运谷胱甘肽(GSH),主要的细胞活性氧(ROS)清除剂的有趣能力,以及它参与上皮到间质转化(EMT)的过程,其中细胞的氧化状态是至关重要的一步。首先,在HEK293-WT细胞中 我们表明低渗条件诱导LRRC8 / VRAC依赖性GSH电导(PGSH / PCl为〜0.1),并且细胞内GSH含量显着降低。GSH电流和GSH细胞内减少都被DCPIB(LRRC8 / VRAC的抑制剂)抑制,在HEK293-LRRC8A KO细胞中未观察到。然后,我们通过将肾小管上皮细胞暴露于多效性生长因子TGFβ1来诱导EMT,并通过测量(i)EMT标记物表达(在基因和蛋白质水平上进行评估)来测量LRRC8 / VRAC在此过程中的贡献, (ii)细胞形态和(iii)迁移能力的提高。有趣的是,药理靶向LRRC8 / VRAC(DCPIB)或RNA干扰介导的抑制(LRRC8A siRNA)通过控制GSH和ROS水平减弱了TGFβ1诱导的EMT反应。有趣的是,TGFβ1的暴露触发了DCPIB敏感的氯化物电导。这些结果表明,LRRC8 / VRAC由于其对GSH的天然渗透性,因而具有调节ROS水平的能力,因此在EMT中起关键作用,并可能有助于与氧化应激相关的其他生理和病理生理过程。









































 京公网安备 11010802027423号
京公网安备 11010802027423号