当前位置:
X-MOL 学术
›
npj Precis. Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synchronous inhibition of mTOR and VEGF/NRP1 axis impedes tumor growth and metastasis in renal cancer.
npj Precision Oncology ( IF 6.8 ) Pub Date : 2019-12-05 , DOI: 10.1038/s41698-019-0105-2 Krishnendu Pal 1 , Vijay Sagar Madamsetty 1 , Shamit Kumar Dutta 1 , Enfeng Wang 1 , Ramcharan Singh Angom 1 , Debabrata Mukhopadhyay 1
npj Precision Oncology ( IF 6.8 ) Pub Date : 2019-12-05 , DOI: 10.1038/s41698-019-0105-2 Krishnendu Pal 1 , Vijay Sagar Madamsetty 1 , Shamit Kumar Dutta 1 , Enfeng Wang 1 , Ramcharan Singh Angom 1 , Debabrata Mukhopadhyay 1
Affiliation
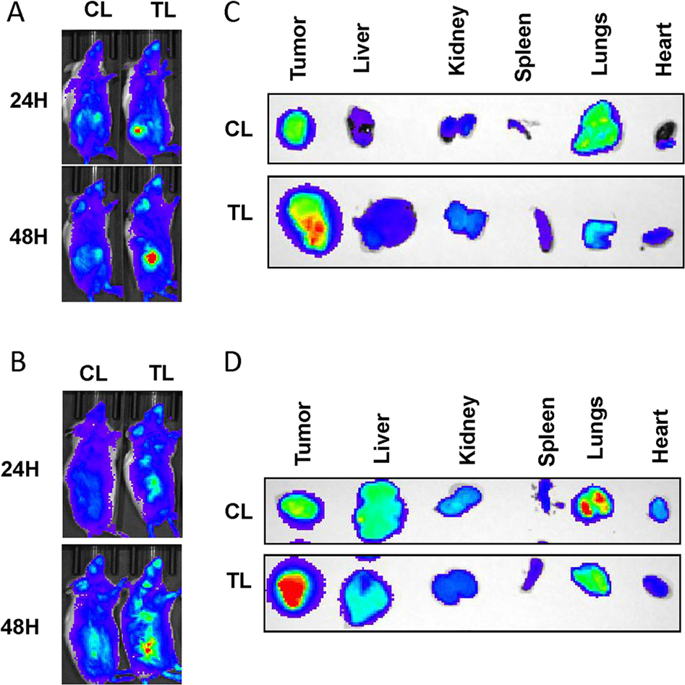
|
Clear cell renal cell carcinoma (ccRCC) is known for its highly vascular phenotype which is associated with elevated expression of vascular endothelial growth factor A (VEGF), also known as vascular permeability factor (VPF). Accordingly, VEGF has been an attractive target for antiangiogenic therapies in ccRCC. Two major strategies have hitherto been utilized for VEGF-targeted antiangiogenic therapies: targeting VEGF by antibodies, ligand traps or aptamers, and targeting the VEGF receptor signaling via antibodies or small-molecule tyrosine-kinase inhibitors (TKIs). In the present article we utilized two entirely different approaches: targeting mammalian target of rapamycin (mTOR) pathway that is known to be involved in VEGF synthesis, and disruption of VEGF/Neuroplin-1 (NRP1) axis that is known to activate proangiogenic and pro-tumorigenic signaling in endothelial and tumor cells, respectively. Everolimus (E) and a small-molecule inhibitor EG00229 (G) were used for the inhibition of mTOR and the disruption of VEGF/NRP1 axis, respectively. We also exploited a liposomal formulation decorated with a proprietary tumor-targeting-peptide (TTP) to simultaneously deliver these two agents in a tumor-targeted manner. The TTP-liposomes encapsulating both Everolimus and EG00229 (EG-L) demonstrated higher in vitro and in vivo growth retardation than the single drug-loaded liposomes (E-L and G-L) in two different ccRCC models and led to a noticeable reduction in lung metastasis in vivo. In addition, EG-L displayed remarkable inhibition of tumor growth in a highly aggressive syngeneic immune-competent mouse model of ccRCC developed in Balb/c mice. Taken together, this study demonstrates an effective approach to achieve improved therapeutic outcome in ccRCC.
中文翻译:

同步抑制 mTOR 和 VEGF/NRP1 轴可阻碍肾癌的肿瘤生长和转移。
透明细胞肾细胞癌 (ccRCC) 以其高度血管表型而闻名,这与血管内皮生长因子 A (VEGF)(也称为血管通透因子 (VPF))表达升高相关。因此,VEGF 已成为 ccRCC 抗血管生成治疗的一个有吸引力的靶点。迄今为止,两种主要策略已用于 VEGF 靶向抗血管生成治疗:通过抗体、配体陷阱或适体靶向 VEGF,以及通过抗体或小分子酪氨酸激酶抑制剂 (TKI) 靶向 VEGF 受体信号传导。在本文中,我们采用了两种完全不同的方法:针对已知参与 VEGF 合成的哺乳动物雷帕霉素靶点 (mTOR) 通路,以及破坏 VEGF/Neuroplin-1 (NRP1) 轴,已知该轴可激活促血管生成和促血管生成。 -分别在内皮细胞和肿瘤细胞中的致瘤信号传导。依维莫司 (E) 和小分子抑制剂 EG00229 (G) 分别用于抑制 mTOR 和破坏 VEGF/NRP1 轴。我们还开发了一种装饰有专有肿瘤靶向肽(TTP)的脂质体制剂,以肿瘤靶向方式同时递送这两种药物。在两种不同的 ccRCC 模型中,封装依维莫司和 EG00229 (EG-L) 的 TTP 脂质体表现出比单一载药脂质体(EL 和 GL)更高的体外和体内生长迟缓,并导致肺转移显着减少。体内。此外,EG-L 在 Balb/c 小鼠中开发的高度侵袭性同基因免疫活性 ccRCC 小鼠模型中显示出对肿瘤生长的显着抑制作用。综上所述,本研究展示了一种改善 ccRCC 治疗效果的有效方法。
更新日期:2019-12-05
中文翻译:

同步抑制 mTOR 和 VEGF/NRP1 轴可阻碍肾癌的肿瘤生长和转移。
透明细胞肾细胞癌 (ccRCC) 以其高度血管表型而闻名,这与血管内皮生长因子 A (VEGF)(也称为血管通透因子 (VPF))表达升高相关。因此,VEGF 已成为 ccRCC 抗血管生成治疗的一个有吸引力的靶点。迄今为止,两种主要策略已用于 VEGF 靶向抗血管生成治疗:通过抗体、配体陷阱或适体靶向 VEGF,以及通过抗体或小分子酪氨酸激酶抑制剂 (TKI) 靶向 VEGF 受体信号传导。在本文中,我们采用了两种完全不同的方法:针对已知参与 VEGF 合成的哺乳动物雷帕霉素靶点 (mTOR) 通路,以及破坏 VEGF/Neuroplin-1 (NRP1) 轴,已知该轴可激活促血管生成和促血管生成。 -分别在内皮细胞和肿瘤细胞中的致瘤信号传导。依维莫司 (E) 和小分子抑制剂 EG00229 (G) 分别用于抑制 mTOR 和破坏 VEGF/NRP1 轴。我们还开发了一种装饰有专有肿瘤靶向肽(TTP)的脂质体制剂,以肿瘤靶向方式同时递送这两种药物。在两种不同的 ccRCC 模型中,封装依维莫司和 EG00229 (EG-L) 的 TTP 脂质体表现出比单一载药脂质体(EL 和 GL)更高的体外和体内生长迟缓,并导致肺转移显着减少。体内。此外,EG-L 在 Balb/c 小鼠中开发的高度侵袭性同基因免疫活性 ccRCC 小鼠模型中显示出对肿瘤生长的显着抑制作用。综上所述,本研究展示了一种改善 ccRCC 治疗效果的有效方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号