当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Analysis of in Vivo Methionine Oxidation of the Human Proteome.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jproteome.9b00505 John Q Bettinger 1 , Kevin A Welle 2 , Jennifer R Hryhorenko 2 , Sina Ghaemmaghami 1, 2
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jproteome.9b00505 John Q Bettinger 1 , Kevin A Welle 2 , Jennifer R Hryhorenko 2 , Sina Ghaemmaghami 1, 2
Affiliation
|
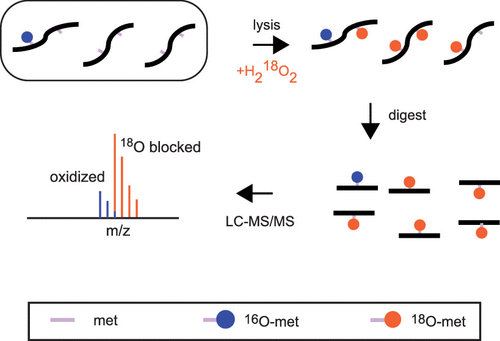
The oxidation of methionine is an important post-translational modification of proteins with numerous roles in physiology and pathology. However, the quantitative analysis of methionine oxidation on a proteome-wide scale has been hampered by technical limitations. Methionine is readily oxidized in vitro during sample preparation and analysis. In addition, there is a lack of enrichment protocols for peptides that contain an oxidized methionine residue, making the accurate quantification of methionine oxidation difficult to achieve on a global scale. Herein, we report a methodology to circumvent these issues by isotopically labeling unoxidized methionines with 18O-labeled hydrogen peroxide and quantifying the relative ratios of 18O- and 16O-oxidized methionines. We validate our methodology using artificially oxidized proteomes made to mimic varying degrees of methionine oxidation. Using this method, we identify and quantify a number of novel sites of in vivo methionine oxidation in an unstressed human cell line.
中文翻译:

人体蛋白质组体内蛋氨酸氧化的定量分析。
蛋氨酸的氧化是蛋白质的重要翻译后修饰,在生理和病理学中具有多种作用。但是,由于蛋白质的局限性,无法在蛋白质组范围内对蛋氨酸氧化进行定量分析。在样品制备和分析过程中,蛋氨酸在体外容易被氧化。另外,对于包含氧化的蛋氨酸残基的肽缺乏富集方案,这使得难以在全球范围内精确地定量蛋氨酸的氧化。在这里,我们报告了一种通过18O标记的过氧化氢同位素标记未氧化蛋氨酸并定量18O和16O氧化蛋氨酸的相对比例来规避这些问题的方法。我们使用人工氧化的蛋白质组来模拟蛋氨酸氧化程度的不同来验证我们的方法。使用这种方法,我们确定并量化在无压力的人类细胞系中体内甲硫氨酸氧化的许多新位点。
更新日期:2020-01-07
中文翻译:

人体蛋白质组体内蛋氨酸氧化的定量分析。
蛋氨酸的氧化是蛋白质的重要翻译后修饰,在生理和病理学中具有多种作用。但是,由于蛋白质的局限性,无法在蛋白质组范围内对蛋氨酸氧化进行定量分析。在样品制备和分析过程中,蛋氨酸在体外容易被氧化。另外,对于包含氧化的蛋氨酸残基的肽缺乏富集方案,这使得难以在全球范围内精确地定量蛋氨酸的氧化。在这里,我们报告了一种通过18O标记的过氧化氢同位素标记未氧化蛋氨酸并定量18O和16O氧化蛋氨酸的相对比例来规避这些问题的方法。我们使用人工氧化的蛋白质组来模拟蛋氨酸氧化程度的不同来验证我们的方法。使用这种方法,我们确定并量化在无压力的人类细胞系中体内甲硫氨酸氧化的许多新位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号