Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.38287 Jingjing Sun 1, 2 , Yichao Chen 1, 2 , Jieni Xu 1, 2 , Xiangping Song 3, 4 , Zhuoya Wan 1, 2 , Yuqian Du 1, 2 , Weina Ma 1, 2 , Xizhen Li 1, 2 , Lin Zhang 3, 4 , Song Li 1, 2
|
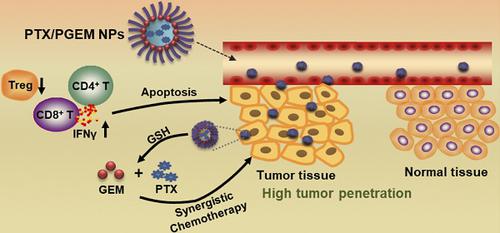
Development of small-sized nanoformulations for effective tumor penetration, particularly for those tumors with dense stroma is a major challenge in cancer nanomedicine. It is even more challenging to achieve effective co-loading of both hydrophobic and hydrophilic anticancer agents through a small-sized nanocarrier. In this work, we designed a novel redox-responsive gemcitabine (GEM)-conjugated polymer POEG-co-PVDGEM (PGEM) as a small-sized nanocarrier to co-deliver hydrophilic GEM and hydrophobic paclitaxel (PTX).
Methods: The in vitro physicochemical and biological properties of PTX/PGEM NPs were characterized. The efficiency of the PGEM carrier in selective codelivery of GEM and PTX in two murine tumor models as well as a patient derived xenograft model (PDX) was also evaluated. In addition, we investigated the changes in tumor immune microenvironment after treatment with PTX/PGEM nanoparticles.
Results: We discovered that GEM conjugation could significantly decrease the nanoparticle size from 160 nm to 13 nm. Moreover, different from most reported GEM-conjugated polymers, PGEM polymer could serve as a prodrug carrier to load a wide variety of hydrophobic agents with high drug loading capacity and excellent stability. More importantly, our strategy could be extended to various nucleotides-based drugs such as azacytidine, decitabine and cytarabine, suggesting a new platform for co-delivery of various first line hydrophilic and hydrophobic anticancer agents. Imaging showed that our small-sized carrier was much more effective in tumor accumulation and penetration compared to the relatively large-sized drug carrier. The PGEM prodrug-based carrier not only well retained the pharmacological activity of GEM, but also boosted T-cell immune response. Furthermore, delivery of PTX via PGEM led to significantly improved antitumor activity in several murine cancer models and a PDX model of colon cancer.
Conclusion: This work not only provided a small-sized carrier platform that was able to load multiple hydrophilic and hydrophobic drugs with high loading capacity, but also provided an effective regimen for enhanced tumor penetration and improved anti-tumor immunity.
中文翻译:

通过小型免疫刺激载体高负载疏水性和亲水性试剂,以增强肿瘤渗透和联合治疗。
开发有效渗透肿瘤的小尺寸纳米制剂,特别是对于那些具有致密基质的肿瘤,是癌症纳米医学的主要挑战。通过小尺寸纳米载体实现疏水性和亲水性抗癌药物的有效共同负载更具挑战性。在这项工作中,我们设计了一种新型氧化还原响应性吉西他滨(GEM)共轭聚合物POEG-co-PVDGEM(PGEM)作为小尺寸纳米载体,共同递送亲水性GEM和疏水性紫杉醇(PTX)。
方法:对 PTX/PGEM NP 的体外理化和生物学特性进行表征。还评估了 PGEM 载体在两种小鼠肿瘤模型以及患者来源的异种移植模型 (PDX) 中选择性共递送 GEM 和 PTX 的效率。此外,我们还研究了PTX/PGEM纳米颗粒治疗后肿瘤免疫微环境的变化。
结果:我们发现 GEM 共轭可以将纳米颗粒的尺寸从 160 nm 显着减小到 13 nm。此外,与大多数报道的GEM共轭聚合物不同,PGEM聚合物可以作为前药载体负载多种疏水剂,具有高载药量和优异的稳定性。更重要的是,我们的策略可以扩展到各种基于核苷酸的药物,例如氮胞苷、地西他滨和阿糖胞苷,这为共同递送各种一线亲水性和疏水性抗癌药物提供了一个新平台。成像显示,与相对较大尺寸的药物载体相比,我们的小型载体在肿瘤积累和渗透方面更有效。基于PGEM前药的载体不仅很好地保留了GEM的药理活性,而且还增强了T细胞免疫反应。此外,通过 PGEM 递送 PTX 可以显着提高几种小鼠癌症模型和结肠癌 PDX 模型的抗肿瘤活性。
结论:该工作不仅提供了一个能够负载多种亲水性和疏水性药物的高负载能力的小型载体平台,而且为增强肿瘤渗透性和提高抗肿瘤免疫力提供了有效的方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号