Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.37851 Maria Tsioumpekou 1, 2, 3 , Sara I Cunha 3, 4 , Haisha Ma 3, 5 , Aive Åhgren 3 , Jessica Cedervall 1 , Anna-Karin Olsson 1 , Carl-Henrik Heldin 1, 3 , Johan Lennartsson 2, 3
|
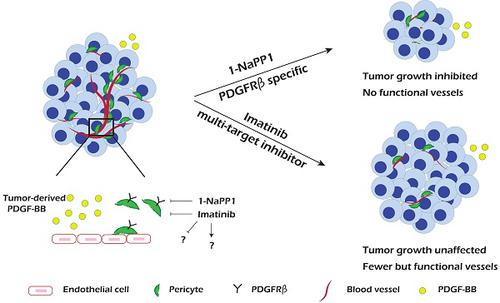
PDGF-BB/PDGFRβ signaling plays an important role during vascularization by mediating pericyte recruitment to the vasculature, promoting the integrity and function of vessels. Until now it has not been possible to assess the specific role of PDGFRβ signaling in tumor progression and angiogenesis due to lack of appropriate animal models and molecular tools.
Methods: In the present study, we used a transgenic knock-in mouse strain carrying a silent mutation in the PDGFRβ ATP binding site that allows specific targeting of PDGFRβ using the compound 1-NaPP1. To evaluate the impact of selective PDGFRβ inhibition of stromal cells on tumor growth we investigated four tumor cell lines with no or low PDGFRβ expression, i.e. Lewis lung carcinoma (LLC), EO771 breast carcinoma, B16 melanoma and a version of B16 that had been engineered to overexpress PDGF-BB (B16/PDGF-BB).
Results: We found that specific impairment of PDGFRβ kinase activity by 1-NaPP1 treatment efficiently suppressed growth in tumors with high expression of PDGF-BB, i.e. LLC and B16/PDGF-BB, while the clinically used PDGFRβ kinase inhibitor imatinib did not suppress tumor growth. Notably, tumors with low levels of PDGF-BB, i.e. EO771 and B16, neither responded to 1-NaPP1 nor to imatinib treatment. Inhibition of PDGFRβ by either drug impaired tumor vascularization and also affected pericyte coverage; however, specific targeting of PDGFRβ by 1-NaPP1 resulted in a more pronounced decrease in vessel function with increased vessel apoptosis in high PDGF-BB expressing tumors, compared to treatment with imatinib. In vitro analysis of PDGFRβ ASKA mouse embryo fibroblasts and the mesenchymal progenitor cell line 10T1/2 revealed that PDGF-BB induced NG2 expression, consistent with the in vivo data.
Conclusion: Specific targeting of PDGFRβ signaling significantly inhibits tumor progression and angiogenesis depending on PDGF-BB expression. Our data suggest that targeting PDGFRβ in the tumor stroma could have therapeutic value in patients with high tumor PDGF-BB expression.
中文翻译:

PDGFRβ在基质中的特异性靶向抑制具有高PDGF-BB表达的肿瘤的生长和血管生成。
PDGF-BB /PDGFRβ信号传导通过介导周细胞募集到脉管系统,促进血管的完整性和功能在血管化过程中发挥重要作用。迄今为止,由于缺乏合适的动物模型和分子工具,尚无法评估PDGFRβ信号传导在肿瘤进展和血管生成中的特定作用。
方法:在本研究中,我们使用了在PDGFRβATP结合位点携带沉默突变的转基因敲入小鼠品系,该化合物允许使用化合物1-NaPP1特异性靶向PDGFRβ。为了评估对基质细胞选择性PDGFRβ抑制对肿瘤生长的影响,我们研究了PDGFRβ表达没有或较低的四种肿瘤细胞系,即。Lewis肺癌(LLC),EO771乳腺癌,B16黑色素瘤和经过工程改造以过表达PDGF-BB(B16 / PDGF-BB)的B16版本。
结果:我们发现1-NaPP1处理对PDGFRβ激酶活性的特定损伤有效地抑制了PDGF-BB高表达的肿瘤(即LLC和B16 / PDGF-BB)的生长,而临床上使用的PDGFRβ激酶抑制剂伊马替尼不能抑制肿瘤生长。值得注意的是,PDGF-BB水平较低的肿瘤,即EO771和B16,对1-NaPP1或伊马替尼治疗均无反应。药物对PDGFRβ的抑制会损害肿瘤的血管形成,并会影响周细胞的覆盖;然而,与伊马替尼治疗相比,1-NaPP1对PDGFRβ的特异性靶向在高PDGF-BB表达的肿瘤中导致血管功能更明显下降,血管凋亡增加。体外PDGFRβASKA小鼠胚胎成纤维细胞和间充质祖细胞系10T1 / 2的分析表明,PDGF-BB诱导NG2表达,与体内数据一致。
结论:取决于PDGF-BB的表达,针对PDGFRβ信号转导的特异性靶向显着抑制肿瘤进展和血管生成。我们的数据表明,在肿瘤基质中靶向PDGFRβ对高肿瘤PDGF-BB表达的患者可能具有治疗价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号