当前位置:
X-MOL 学术
›
Mucosal Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antibiotic-induced microbiome perturbations are associated with significant alterations to colonic mucosal immunity in rhesus macaques.
Mucosal Immunology ( IF 7.9 ) Pub Date : 2019-12-04 , DOI: 10.1038/s41385-019-0238-1 Jennifer A Manuzak 1, 2, 3 , Alexander S Zevin 1, 2 , Ryan Cheu 1, 2, 3 , Brian Richardson 4 , Jacob Modesitt 1, 2 , Tiffany Hensley-McBain 1, 2 , Charlene Miller 1, 2, 3 , Andrew T Gustin 1, 2, 5 , Ernesto Coronado 1, 2 , Toni Gott 1, 2 , Mike Fang 4 , Michael Cartwright 4 , Solomon Wangari 2 , Brian Agricola 2 , Drew May 2 , Elise Smith 5 , Hans Benjamin Hampel 6, 7 , Michael Gale 2, 5 , Cheryl M Cameron 8 , Mark J Cameron 4 , Jeremy Smedley 2, 9 , Nichole R Klatt 1, 2, 3
Mucosal Immunology ( IF 7.9 ) Pub Date : 2019-12-04 , DOI: 10.1038/s41385-019-0238-1 Jennifer A Manuzak 1, 2, 3 , Alexander S Zevin 1, 2 , Ryan Cheu 1, 2, 3 , Brian Richardson 4 , Jacob Modesitt 1, 2 , Tiffany Hensley-McBain 1, 2 , Charlene Miller 1, 2, 3 , Andrew T Gustin 1, 2, 5 , Ernesto Coronado 1, 2 , Toni Gott 1, 2 , Mike Fang 4 , Michael Cartwright 4 , Solomon Wangari 2 , Brian Agricola 2 , Drew May 2 , Elise Smith 5 , Hans Benjamin Hampel 6, 7 , Michael Gale 2, 5 , Cheryl M Cameron 8 , Mark J Cameron 4 , Jeremy Smedley 2, 9 , Nichole R Klatt 1, 2, 3
Affiliation
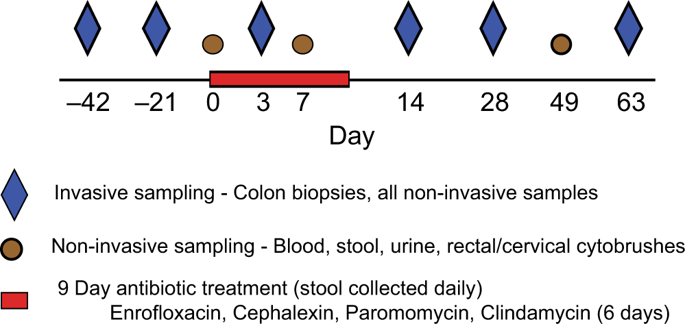
|
The diverse bacterial communities that colonize the gastrointestinal tract play an essential role in maintaining immune homeostasis through the production of critical metabolites such as short-chain fatty acids (SCFAs) and this can be disrupted by antibiotic use. However, few studies have addressed the effects of specific antibiotics longitudinally on the microbiome and immunity. We evaluated the effects of four specific antibiotics: enrofloxacin, cephalexin, paromomycin, and clindamycin, in healthy female rhesus macaques. All antibiotics disrupted the microbiome, including reduced abundances of fermentative bacteria and increased abundances of potentially pathogenic bacteria, including Enterobacteriaceae in the stool, and decreased Helicobacteraceae in the colon. This was associated with decreased SCFAs, indicating altered bacterial metabolism. Importantly, antibiotic use also substantially altered local immune responses, including increased neutrophils and Th17 cells in the colon. Furthermore, we observed increased soluble CD14 in plasma, indicating microbial translocation. These data provide a longitudinal evaluation of antibiotic-induced changes to the composition and function of colonic bacterial communities associated with specific alterations in mucosal and systemic immunity.
中文翻译:

抗生素引起的微生物组扰动与恒河猴结肠粘膜免疫的显着改变有关。
通过产生短链脂肪酸 (SCFA) 等关键代谢物,定植于胃肠道的各种细菌群落在维持免疫稳态方面发挥着重要作用,而抗生素的使用可能会破坏这一点。然而,很少有研究探讨特定抗生素对微生物组和免疫力的纵向影响。我们评估了四种特定抗生素:恩诺沙星、头孢氨苄、巴龙霉素和克林霉素对健康雌性恒河猴的影响。所有抗生素都会破坏微生物组,包括减少发酵细菌的丰度和增加潜在致病菌的丰度,包括粪便中的肠杆菌科,以及减少结肠中的螺杆菌。这与 SCFA 减少有关,表明细菌代谢发生了改变。重要的是,抗生素的使用还显着改变了局部免疫反应,包括结肠中嗜中性粒细胞和 Th17 细胞的增加。此外,我们观察到血浆中可溶性 CD14 增加,表明微生物易位。这些数据提供了对抗生素引起的结肠细菌群落组成和功能变化的纵向评估,这些变化与粘膜和全身免疫的特定改变相关。
更新日期:2019-12-04
中文翻译:

抗生素引起的微生物组扰动与恒河猴结肠粘膜免疫的显着改变有关。
通过产生短链脂肪酸 (SCFA) 等关键代谢物,定植于胃肠道的各种细菌群落在维持免疫稳态方面发挥着重要作用,而抗生素的使用可能会破坏这一点。然而,很少有研究探讨特定抗生素对微生物组和免疫力的纵向影响。我们评估了四种特定抗生素:恩诺沙星、头孢氨苄、巴龙霉素和克林霉素对健康雌性恒河猴的影响。所有抗生素都会破坏微生物组,包括减少发酵细菌的丰度和增加潜在致病菌的丰度,包括粪便中的肠杆菌科,以及减少结肠中的螺杆菌。这与 SCFA 减少有关,表明细菌代谢发生了改变。重要的是,抗生素的使用还显着改变了局部免疫反应,包括结肠中嗜中性粒细胞和 Th17 细胞的增加。此外,我们观察到血浆中可溶性 CD14 增加,表明微生物易位。这些数据提供了对抗生素引起的结肠细菌群落组成和功能变化的纵向评估,这些变化与粘膜和全身免疫的特定改变相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号