当前位置:
X-MOL 学术
›
Cell Death Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway.
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2019-12-04 , DOI: 10.1038/s41418-019-0460-0 Chao Liu 1, 2 , Zhaoying Yao 1, 2 , Jianing Wang 3 , Wen Zhang 1, 2 , Yan Yang 4 , Yan Zhang 5 , Xinliang Qu 6 , Yubing Zhu 1, 2 , Jianjun Zou 1, 2 , Sishi Peng 2, 7 , Yan Zhao 1, 2 , Shuli Zhao 7 , Bangshun He 7 , Qiongyu Mi 7 , Xiuting Liu 8 , Xu Zhang 9, 10, 11 , Qianming Du 7
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2019-12-04 , DOI: 10.1038/s41418-019-0460-0 Chao Liu 1, 2 , Zhaoying Yao 1, 2 , Jianing Wang 3 , Wen Zhang 1, 2 , Yan Yang 4 , Yan Zhang 5 , Xinliang Qu 6 , Yubing Zhu 1, 2 , Jianjun Zou 1, 2 , Sishi Peng 2, 7 , Yan Zhao 1, 2 , Shuli Zhao 7 , Bangshun He 7 , Qiongyu Mi 7 , Xiuting Liu 8 , Xu Zhang 9, 10, 11 , Qianming Du 7
Affiliation
|
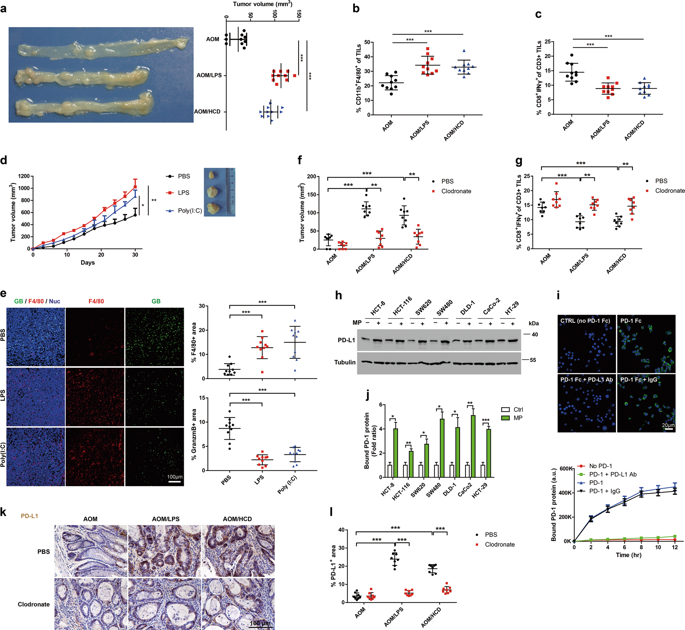
Infiltrated macrophages are an important constituent of the tumor microenvironment and play roles in tumor initiation and progression by promoting immune evasion. However, the molecular mechanism by which macrophage-derived cytokines foster immune escape of colorectal cancer (CRC) is unclear. Here, we demonstrated that macrophage infiltration induced by lipopolysaccharide (LPS) or a high-cholesterol diet (HCD) significantly promoted CRC growth. Similarly, LPS and poly (I:C) remarkably increased the volume of CT26 cell allograft tumors. C-C motif chemokine ligand 5 (CCL5), which is secreted by macrophages, inhibited T-cell-mediated killing of HT29 cells and promoted immune escape by stabilizing PD-L1 in vitro and in vivo. Mechanistically, CCL5 resulted in formation of nuclear factor kappa-B p65/STAT3 complexes, which bound to the COP9 signalosome 5 (CSN5) promoter, leading to its upregulation. Moreover, CSN5 modulated the deubiquitination and stability of PD-L1. High expression of CSN5 in CRC was associated with significantly shorter survival. Furthermore, compound-15 was identified as an inhibitor of CSN5, and destabilized PD-L1 to alleviate the tumor burden. Our results suggest that the novel CCL5-p65/STAT3-CSN5-PD-L1 signaling axis is significantly activated by LPS or HCD-driven macrophage infiltration in an animal model of CRC, which likely has therapeutic and prognostic implications for human cancers.
中文翻译:

巨噬细胞衍生的 CCL5 通过 p65/STAT3-CSN5-PD-L1 通路促进结直肠癌细胞的免疫逃逸。
浸润的巨噬细胞是肿瘤微环境的重要组成部分,通过促进免疫逃避而在肿瘤的发生和发展中发挥作用。然而,巨噬细胞衍生细胞因子促进结直肠癌 (CRC) 免疫逃逸的分子机制尚不清楚。在这里,我们证明了由脂多糖 (LPS) 或高胆固醇饮食 (HCD) 诱导的巨噬细胞浸润显着促进了 CRC 的生长。同样,LPS 和 poly (I:C) 显着增加了 CT26 细胞同种异体移植肿瘤的体积。由巨噬细胞分泌的 CC 基序趋化因子配体 5 (CCL5) 通过在体外和体内稳定 PD-L1 抑制 T 细胞介导的 HT29 细胞杀伤并促进免疫逃逸。从机制上讲,CCL5 导致核因子 kappa-B p65/STAT3 复合物的形成,它与 COP9 信号体 5 (CSN5) 启动子结合,导致其上调。此外,CSN5 调节了 PD-L1 的去泛素化和稳定性。CSN5 在 CRC 中的高表达与显着缩短的生存期相关。此外,化合物 15 被鉴定为 CSN5 的抑制剂,并使 PD-L1 不稳定以减轻肿瘤负担。我们的研究结果表明,新的 CCL5-p65/STAT3-CSN5-PD-L1 信号轴在 CRC 动物模型中被 LPS 或 HCD 驱动的巨噬细胞浸润显着激活,这可能对人类癌症具有治疗和预后意义。化合物 15 被确定为 CSN5 的抑制剂,并破坏 PD-L1 以减轻肿瘤负担。我们的研究结果表明,新的 CCL5-p65/STAT3-CSN5-PD-L1 信号轴在 CRC 动物模型中被 LPS 或 HCD 驱动的巨噬细胞浸润显着激活,这可能对人类癌症具有治疗和预后意义。化合物 15 被确定为 CSN5 的抑制剂,并破坏 PD-L1 以减轻肿瘤负担。我们的研究结果表明,新的 CCL5-p65/STAT3-CSN5-PD-L1 信号轴在 CRC 动物模型中被 LPS 或 HCD 驱动的巨噬细胞浸润显着激活,这可能对人类癌症具有治疗和预后意义。
更新日期:2019-12-04
中文翻译:

巨噬细胞衍生的 CCL5 通过 p65/STAT3-CSN5-PD-L1 通路促进结直肠癌细胞的免疫逃逸。
浸润的巨噬细胞是肿瘤微环境的重要组成部分,通过促进免疫逃避而在肿瘤的发生和发展中发挥作用。然而,巨噬细胞衍生细胞因子促进结直肠癌 (CRC) 免疫逃逸的分子机制尚不清楚。在这里,我们证明了由脂多糖 (LPS) 或高胆固醇饮食 (HCD) 诱导的巨噬细胞浸润显着促进了 CRC 的生长。同样,LPS 和 poly (I:C) 显着增加了 CT26 细胞同种异体移植肿瘤的体积。由巨噬细胞分泌的 CC 基序趋化因子配体 5 (CCL5) 通过在体外和体内稳定 PD-L1 抑制 T 细胞介导的 HT29 细胞杀伤并促进免疫逃逸。从机制上讲,CCL5 导致核因子 kappa-B p65/STAT3 复合物的形成,它与 COP9 信号体 5 (CSN5) 启动子结合,导致其上调。此外,CSN5 调节了 PD-L1 的去泛素化和稳定性。CSN5 在 CRC 中的高表达与显着缩短的生存期相关。此外,化合物 15 被鉴定为 CSN5 的抑制剂,并使 PD-L1 不稳定以减轻肿瘤负担。我们的研究结果表明,新的 CCL5-p65/STAT3-CSN5-PD-L1 信号轴在 CRC 动物模型中被 LPS 或 HCD 驱动的巨噬细胞浸润显着激活,这可能对人类癌症具有治疗和预后意义。化合物 15 被确定为 CSN5 的抑制剂,并破坏 PD-L1 以减轻肿瘤负担。我们的研究结果表明,新的 CCL5-p65/STAT3-CSN5-PD-L1 信号轴在 CRC 动物模型中被 LPS 或 HCD 驱动的巨噬细胞浸润显着激活,这可能对人类癌症具有治疗和预后意义。化合物 15 被确定为 CSN5 的抑制剂,并破坏 PD-L1 以减轻肿瘤负担。我们的研究结果表明,新的 CCL5-p65/STAT3-CSN5-PD-L1 信号轴在 CRC 动物模型中被 LPS 或 HCD 驱动的巨噬细胞浸润显着激活,这可能对人类癌症具有治疗和预后意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号