当前位置:
X-MOL 学术
›
Appl. Clay. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Removal of cationic methylene blue dye using magnetic and anionic-cationic modified montmorillonite: kinetic, isotherm and thermodynamic studies
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.clay.2019.105391 Soleiman Rahmani , Behzad Zeynizadeh , Shiva Karami
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.clay.2019.105391 Soleiman Rahmani , Behzad Zeynizadeh , Shiva Karami
|
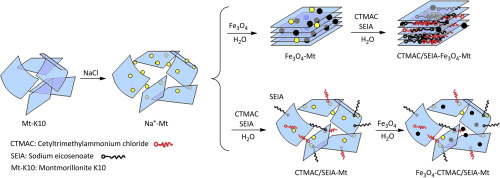
Abstract In this study, the synthesis of new class of the modified montmorillonite with magnetic nanoparticles and surfactants was studied. The synthesis was carried out via intercalation of a mixture of sodium eicosenoate (SEIA, anionic surfactant) and cetyltrimethylammonium chloride (CTMAC, cationic surfactant) on the surface or within interlamellar spaces of montmorillonite (Mt) and thereafter the immobilization of Fe3O4 MNPs (magnetic nanoparticles) to afford Fe3O4-CTMAC/SEIA-Mt system. Next, the nanocomposite of CTMAC-Mt, SEIA-Mt, CTMAC/SEIA-Mt and CTMAC/SEIA-Fe3O4-Mt (In this case, magnetic montmorillonite was primarily prepared and then modified with a mixture of CTMAC/SEIA surfactants) were also synthesized. The prepared nanocomposite systems were characterized using FT-IR, XRD, SEM, EDX, TGA and VSM analyses and then utilized for the removal of cationic methylene blue (MB) dye from aqueous solutions. Among the examined composite systems, Fe3O4-CTMAC/SEIA-Mt showed a superior adsorption capacity. In this area, the influence of adsorbent dosage, contact time, initial concentration of MB and temperature towards the adsorption of MB on Fe3O4-CTMAC/SEIA-Mt system was also investigated. The obtained results exhibited that the kinetic of adsorption and the model of isotherm obeyed from the pseudo-second order and Langmuir model, respectively. Moreover, Fe3O4-CTMAC/SEIA-Mt system showed an extraordinary adsorption capacity for MB (246 mg g–1). Because of superparamagnetic characteristic of magnetite, the adsorbent of Fe3O4-CTMAC/SEIA-Mt can be easily separated from the reaction mixture by an external magnetic field. Thermodynamic studies also represented that the adsorption of MB on Fe3O4-CTMAC/SEIA-Mt was endothermic and it was carried out as a spontaneous process.
中文翻译:

使用磁性和阴离子-阳离子改性蒙脱石去除阳离子亚甲蓝染料:动力学、等温线和热力学研究
摘要 本研究对新型磁性纳米颗粒和表面活性剂改性蒙脱石的合成进行了研究。该合成是通过在蒙脱石 (Mt) 的表面或层间空间内嵌入二十碳烯酸钠(SEIA,阴离子表面活性剂)和十六烷基三甲基氯化铵(CTMAC,阳离子表面活性剂)的混合物进行的,然后固定 Fe3O4 MNP(磁性纳米颗粒) ) 得到 Fe3O4-CTMAC/SEIA-Mt 系统。接下来,CTMAC-Mt、SEIA-Mt、CTMAC/SEIA-Mt 和 CTMAC/SEIA-Fe3O4-Mt 的纳米复合材料(在这种情况下,首先制备磁性蒙脱石,然后用 CTMAC/SEIA 表面活性剂的混合物改性)合成的。使用 FT-IR、XRD、SEM、EDX、TGA 和 VSM 分析,然后用于从水溶液中去除阳离子亚甲蓝 (MB) 染料。在所检测的复合系统中,Fe3O4-CTMAC/SEIA-Mt 表现出优异的吸附能力。在该领域,还研究了吸附剂用量、接触时间、MB 初始浓度和温度对 MB 在 Fe3O4-CTMAC/SEIA-Mt 系统上吸附的影响。所得结果表明吸附动力学和等温线模型分别符合伪二级和朗缪尔模型。此外,Fe3O4-CTMAC/SEIA-Mt 系统对 MB (246 mg g-1) 显示出非凡的吸附能力。由于磁铁矿的超顺磁性特性,Fe3O4-CTMAC/SEIA-Mt 的吸附剂可以很容易地通过外部磁场从反应混合物中分离出来。
更新日期:2020-01-01
中文翻译:

使用磁性和阴离子-阳离子改性蒙脱石去除阳离子亚甲蓝染料:动力学、等温线和热力学研究
摘要 本研究对新型磁性纳米颗粒和表面活性剂改性蒙脱石的合成进行了研究。该合成是通过在蒙脱石 (Mt) 的表面或层间空间内嵌入二十碳烯酸钠(SEIA,阴离子表面活性剂)和十六烷基三甲基氯化铵(CTMAC,阳离子表面活性剂)的混合物进行的,然后固定 Fe3O4 MNP(磁性纳米颗粒) ) 得到 Fe3O4-CTMAC/SEIA-Mt 系统。接下来,CTMAC-Mt、SEIA-Mt、CTMAC/SEIA-Mt 和 CTMAC/SEIA-Fe3O4-Mt 的纳米复合材料(在这种情况下,首先制备磁性蒙脱石,然后用 CTMAC/SEIA 表面活性剂的混合物改性)合成的。使用 FT-IR、XRD、SEM、EDX、TGA 和 VSM 分析,然后用于从水溶液中去除阳离子亚甲蓝 (MB) 染料。在所检测的复合系统中,Fe3O4-CTMAC/SEIA-Mt 表现出优异的吸附能力。在该领域,还研究了吸附剂用量、接触时间、MB 初始浓度和温度对 MB 在 Fe3O4-CTMAC/SEIA-Mt 系统上吸附的影响。所得结果表明吸附动力学和等温线模型分别符合伪二级和朗缪尔模型。此外,Fe3O4-CTMAC/SEIA-Mt 系统对 MB (246 mg g-1) 显示出非凡的吸附能力。由于磁铁矿的超顺磁性特性,Fe3O4-CTMAC/SEIA-Mt 的吸附剂可以很容易地通过外部磁场从反应混合物中分离出来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号