当前位置:
X-MOL 学术
›
Sleep Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measures of functional outcomes, work productivity, and quality of life from a randomized, phase 3 study of solriamfetol in participants with narcolepsy.
Sleep Medicine ( IF 3.8 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.sleep.2019.11.1250 Helene A Emsellem 1 , Michael J Thorpy 2 , Gert Jan Lammers 3 , Colin M Shapiro 4 , Geert Mayer 5 , Giuseppe Plazzi 6 , Dan Chen 7 , Lawrence P Carter 8 , Kathleen F Villa 7 , Lawrence Lee 7 , Diane Menno 9 , Jed Black 10 , Yves Dauvilliers 11
Sleep Medicine ( IF 3.8 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.sleep.2019.11.1250 Helene A Emsellem 1 , Michael J Thorpy 2 , Gert Jan Lammers 3 , Colin M Shapiro 4 , Geert Mayer 5 , Giuseppe Plazzi 6 , Dan Chen 7 , Lawrence P Carter 8 , Kathleen F Villa 7 , Lawrence Lee 7 , Diane Menno 9 , Jed Black 10 , Yves Dauvilliers 11
Affiliation
|
OBJECTIVE
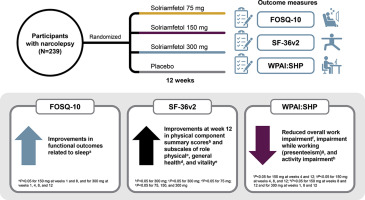
Solriamfetol (formerly JZP-110), a dopamine/norepinephrine reuptake inhibitor, is approved in the US to improve wakefulness in adults with excessive daytime sleepiness associated with narcolepsy (75-150 mg/d) or obstructive sleep apnea (37.5-150 mg/d). In a randomized, double-blind, placebo-controlled trial in participants with narcolepsy, effects of solriamfetol on functional status, health-related quality of life (HRQoL), and work productivity were evaluated.
METHODS
Participants with narcolepsy (N = 239) were randomized to solriamfetol 75, 150, or 300 mg, or placebo for 12 weeks. Outcome measures included the Functional Outcomes of Sleep Questionnaire short version (FOSQ-10), 36-Item Short Form Health Survey version 2 (SF-36v2), and Work Productivity and Activity Impairment questionnaire for Specific Health Problem (WPAI:SHP). A mixed-effects model with repeated measures was used for comparisons vs placebo.
RESULTS
At week 12, solriamfetol increased FOSQ-10 total score, with greatest mean difference from placebo (95% CI) at 300 mg (1.45 [0.31, 2.59]). On SF-36v2, improvements vs placebo were observed in physical component summary scores (300 mg: 2.22 [0.04, 4.41]) and subscales of role physical, general health, and vitality. On WPAI:SHP, solriamfetol 150 mg reduced overall work impairment vs placebo (-15.5 [-29.52, -1.47]), and 150 and 300 mg reduced activity impairment vs placebo (-10.05 [-19.48, -0.62] and -13.49 [-23.19, -3.78], respectively). Most treatment-emergent adverse events (TEAEs) were mild or moderate in severity. Common TEAEs were headache, nausea, decreased appetite, nasopharyngitis, dry mouth, and anxiety.
CONCLUSIONS
Solriamfetol improved measures of functional status, HRQoL, and work productivity, particularly at the 150- and 300-mg doses. Most TEAEs were mild to moderate.
TRIAL REGISTRATION
ClinicalTrials.gov identifier NCT02348593, EudraCT number 2014-005487-15.
中文翻译:

通过对发作性睡病患者进行索拉莫非特的3期随机研究,评估其功能结局,工作效率和生活质量。
目的美国多巴胺/去甲肾上腺素再摄取抑制剂索里亚莫非(原名JZP-110)已获批准,可改善患有嗜睡症(75-150 mg / d)或阻塞性睡眠呼吸暂停(37.5-150 mg)的白天过度嗜睡的成年人的觉醒/ d)。在一项对发作性睡病患者进行的随机,双盲,安慰剂对照试验中,评估了多效酚对功能状态,与健康相关的生活质量(HRQoL)和工作效率的影响。方法发作性睡病患者(N = 239)被随机分配到75、150或300 mg索里亚非尔或安慰剂中,为期12周。成果指标包括功能性睡眠问卷简写(FOSQ-10),36项简明健康调查版本2(SF-36v2),以及针对特定健康问题的工作效率和活动障碍问卷(WPAI:SHP)。与安慰剂相比,使用具有重复测量的混合效应模型进行比较。结果在第12周时,索里亚莫非酚增加FOSQ-10总评分,与安慰剂(95%CI)的最大平均差异为300 mg(1.45 [0.31,2.59])。在SF-36v2上,观察到与安慰剂相比,身体成分摘要评分(300 mg:2.22 [0.04,4.41])以及身体,总体健康和活力的次级量表均得到改善。在WPAI:SHP上,索拉莫非酚150 mg相对于安慰剂减少了总体工作障碍(-15.5 [-29.52,-1.47]),而150和300 mg相对于安慰剂则减少了活动障碍(-10.05 [-19.48,-0.62]和-13.49 [ -23.19,-3.78])。大多数治疗紧急不良事件(TEAE)的严重程度为轻度或中度。常见的TEAE为头痛,恶心,食欲不振,鼻咽炎,口干和焦虑。结论Solriamfetol改善了功能状态,HRQoL和工作效率的量度,尤其是在150和300 mg剂量时。多数TEAE为轻度至中度。试验注册ClinicalTrials.gov标识符NCT02348593,EudraCT号2014-005487-15。
更新日期:2019-12-04
中文翻译:

通过对发作性睡病患者进行索拉莫非特的3期随机研究,评估其功能结局,工作效率和生活质量。
目的美国多巴胺/去甲肾上腺素再摄取抑制剂索里亚莫非(原名JZP-110)已获批准,可改善患有嗜睡症(75-150 mg / d)或阻塞性睡眠呼吸暂停(37.5-150 mg)的白天过度嗜睡的成年人的觉醒/ d)。在一项对发作性睡病患者进行的随机,双盲,安慰剂对照试验中,评估了多效酚对功能状态,与健康相关的生活质量(HRQoL)和工作效率的影响。方法发作性睡病患者(N = 239)被随机分配到75、150或300 mg索里亚非尔或安慰剂中,为期12周。成果指标包括功能性睡眠问卷简写(FOSQ-10),36项简明健康调查版本2(SF-36v2),以及针对特定健康问题的工作效率和活动障碍问卷(WPAI:SHP)。与安慰剂相比,使用具有重复测量的混合效应模型进行比较。结果在第12周时,索里亚莫非酚增加FOSQ-10总评分,与安慰剂(95%CI)的最大平均差异为300 mg(1.45 [0.31,2.59])。在SF-36v2上,观察到与安慰剂相比,身体成分摘要评分(300 mg:2.22 [0.04,4.41])以及身体,总体健康和活力的次级量表均得到改善。在WPAI:SHP上,索拉莫非酚150 mg相对于安慰剂减少了总体工作障碍(-15.5 [-29.52,-1.47]),而150和300 mg相对于安慰剂则减少了活动障碍(-10.05 [-19.48,-0.62]和-13.49 [ -23.19,-3.78])。大多数治疗紧急不良事件(TEAE)的严重程度为轻度或中度。常见的TEAE为头痛,恶心,食欲不振,鼻咽炎,口干和焦虑。结论Solriamfetol改善了功能状态,HRQoL和工作效率的量度,尤其是在150和300 mg剂量时。多数TEAE为轻度至中度。试验注册ClinicalTrials.gov标识符NCT02348593,EudraCT号2014-005487-15。











































 京公网安备 11010802027423号
京公网安备 11010802027423号