当前位置:
X-MOL 学术
›
Mol. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Pharmacological Chaperone Therapy for Acute Intermittent Porphyria.
Molecular Therapy ( IF 12.1 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.ymthe.2019.11.010 Helene J Bustad 1 , Karen Toska 2 , Caroline Schmitt 3 , Marta Vorland 2 , Lars Skjærven 1 , Juha P Kallio 1 , Sylvie Simonin 3 , Philippe Letteron 4 , Jarl Underhaug 5 , Sverre Sandberg 6 , Aurora Martinez 1
Molecular Therapy ( IF 12.1 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.ymthe.2019.11.010 Helene J Bustad 1 , Karen Toska 2 , Caroline Schmitt 3 , Marta Vorland 2 , Lars Skjærven 1 , Juha P Kallio 1 , Sylvie Simonin 3 , Philippe Letteron 4 , Jarl Underhaug 5 , Sverre Sandberg 6 , Aurora Martinez 1
Affiliation
|
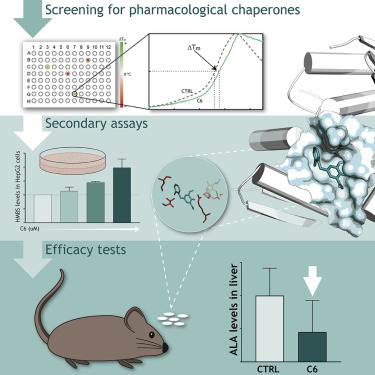
Mutations in hydroxymethylbilane synthase (HMBS) cause acute intermittent porphyria (AIP), an autosomal dominant disease where typically only one HMBS allele is mutated. In AIP, the accumulation of porphyrin precursors triggers life-threatening neurovisceral attacks and at long-term, entails an increased risk of hepatocellular carcinoma, kidney failure, and hypertension. Today, the only cure is liver transplantation, and a need for effective mechanism-based therapies, such as pharmacological chaperones, is prevailing. These are small molecules that specifically stabilize a target protein. They may be developed into an oral treatment, which could work curatively during acute attacks, but also prophylactically in asymptomatic HMBS mutant carriers. With the use of a 10,000 compound library, we identified four binders that further increased the initially very high thermal stability of wild-type HMBS and protected the enzyme from trypsin digestion. The best hit and a selected analog increased steady-state levels and total HMBS activity in human hepatoma cells overexpressing HMBS, and in an Hmbs-deficient mouse model with a low-expressed wild-type-like allele, compared to untreated controls. Moreover, the concentration of porphyrin precursors decreased in liver of mice treated with the best hit. Our findings demonstrate the great potential of these hits for the development of a pharmacological chaperone-based corrective treatment of AIP by enhancing wild-type HMBS function independently of the patients' specific mutation.
中文翻译:

急性间歇性卟啉症的药理伴侣疗法。
羟甲基胆烷合酶(HMBS)中的突变会引起急性间歇性卟啉症(AIP),这是一种常染色体显性疾病,通常只有一个HMBS等位基因发生突变。在AIP中,卟啉前体的积累会触发威胁生命的神经内脏攻击,长期而言,这会增加患肝细胞癌,肾衰竭和高血压的风险。如今,唯一的治疗方法是肝移植,并且对基于有效的基于机制的疗法(例如药理伴侣)的需求日益盛行。这些是特异稳定靶蛋白的小分子。它们可以发展为口服治疗,在急性发作期间可以治愈,但在无症状的HMBS突变携带者中也可以预防性治疗。通过使用10,000个化合物库,我们鉴定了四种结合物,它们进一步提高了野生型HMBS最初的非常高的热稳定性,并保护了酶免于胰蛋白酶的消化。与未经处理的对照相比,最佳命中和选定的类似物增加了过表达HMBS的人肝癌细胞中以及具有低表达的野生型样等位基因的Hmbs缺陷小鼠模型的稳态水平和总HMBS活性。此外,在遭受最佳打击的小鼠的肝脏中,卟啉前体的浓度降低了。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特异性突变,可开发出基于药理伴侣的AIP矫正疗法,具有巨大的潜力。与未经处理的对照相比,最佳命中和选定的类似物增加了过表达HMBS的人肝癌细胞中以及具有低表达的野生型样等位基因的Hmbs缺陷小鼠模型的稳态水平和总HMBS活性。此外,在遭受最佳打击的小鼠的肝脏中,卟啉前体的浓度降低了。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特异性突变,可开发出基于药理伴侣的AIP矫正疗法,具有巨大的潜力。与未经处理的对照相比,最佳命中和选定的类似物增加了过表达HMBS的人肝癌细胞中以及具有低表达的野生型样等位基因的Hmbs缺陷小鼠模型的稳态水平和总HMBS活性。此外,在遭受最佳打击的小鼠的肝脏中,卟啉前体的浓度降低了。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特异性突变,可开发出基于药理伴侣的AIP矫正疗法,具有巨大的潜力。遭受最佳打击的小鼠肝脏中卟啉前体的浓度降低。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特定突变,可开发出基于药理伴侣的纠正性治疗AIP的巨大潜力。遭受最佳打击的小鼠肝脏中卟啉前体的浓度降低。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特异性突变,可开发出基于药理伴侣的AIP矫正疗法,具有巨大的潜力。
更新日期:2019-12-04
中文翻译:

急性间歇性卟啉症的药理伴侣疗法。
羟甲基胆烷合酶(HMBS)中的突变会引起急性间歇性卟啉症(AIP),这是一种常染色体显性疾病,通常只有一个HMBS等位基因发生突变。在AIP中,卟啉前体的积累会触发威胁生命的神经内脏攻击,长期而言,这会增加患肝细胞癌,肾衰竭和高血压的风险。如今,唯一的治疗方法是肝移植,并且对基于有效的基于机制的疗法(例如药理伴侣)的需求日益盛行。这些是特异稳定靶蛋白的小分子。它们可以发展为口服治疗,在急性发作期间可以治愈,但在无症状的HMBS突变携带者中也可以预防性治疗。通过使用10,000个化合物库,我们鉴定了四种结合物,它们进一步提高了野生型HMBS最初的非常高的热稳定性,并保护了酶免于胰蛋白酶的消化。与未经处理的对照相比,最佳命中和选定的类似物增加了过表达HMBS的人肝癌细胞中以及具有低表达的野生型样等位基因的Hmbs缺陷小鼠模型的稳态水平和总HMBS活性。此外,在遭受最佳打击的小鼠的肝脏中,卟啉前体的浓度降低了。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特异性突变,可开发出基于药理伴侣的AIP矫正疗法,具有巨大的潜力。与未经处理的对照相比,最佳命中和选定的类似物增加了过表达HMBS的人肝癌细胞中以及具有低表达的野生型样等位基因的Hmbs缺陷小鼠模型的稳态水平和总HMBS活性。此外,在遭受最佳打击的小鼠的肝脏中,卟啉前体的浓度降低了。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特异性突变,可开发出基于药理伴侣的AIP矫正疗法,具有巨大的潜力。与未经处理的对照相比,最佳命中和选定的类似物增加了过表达HMBS的人肝癌细胞中以及具有低表达的野生型样等位基因的Hmbs缺陷小鼠模型的稳态水平和总HMBS活性。此外,在遭受最佳打击的小鼠的肝脏中,卟啉前体的浓度降低了。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特异性突变,可开发出基于药理伴侣的AIP矫正疗法,具有巨大的潜力。遭受最佳打击的小鼠肝脏中卟啉前体的浓度降低。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特定突变,可开发出基于药理伴侣的纠正性治疗AIP的巨大潜力。遭受最佳打击的小鼠肝脏中卟啉前体的浓度降低。我们的发现表明,这些命中物通过增强野生型HMBS功能而独立于患者的特异性突变,可开发出基于药理伴侣的AIP矫正疗法,具有巨大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号